Answers
Answer:
C₁₂H₂₂O₁₁ + H₂O → C₅H₁₂O₆ + C₆H₁₂O₆
Explanation:
Chemical equation:
C₁₂H₂₂O₁₁ + H₂O → C₅H₁₂O₆ + C₆H₁₂O₆
Source of sucrose:
Sucrose is present in roots of plants and also in fruits. It is storage form of energy. Some insects and bacteria use sucrose as main food. Best example is honeybee which collect sucrose and convert it into honey.
Monomers of sucrose and hydrolysis:
Sucrose consist of monomers glucose and fructose which are join together through glycosidic bond. Hydrolysis break the sucrose molecule into glucose and fructose. In hydrolysis glycosidic bond is break which convert the sucrose into glucose and fructose. Hydrolysis is slow process but this reaction is catalyze by enzyme. The enzyme invertase catalyze this reaction.
The given reaction also completely follow the law of conservation of mass. There are equal number of atoms of elements on both side of chemical equation thus mass remain conserved.
Related Questions
A student mixes 43.8 mL of acetone (58.08 g/mol, 0.791 g/mL) with excess benzaldehyde and NaOH to produce 79.4 g of (1E,4E)-1,5-diphenylpenta-1,4-dien-3-one (234.29 g/mol). What is the percent yield of this student's experiment
Answers
Answer:
% yield of the student's experiment is
\(\frac{0.34}{0.60}\) ˣ 100 = 56.67%
Explanation:
given
volume of acetone= 43.8 mL
molar weight of acetone = 58.08 g/mol
density of acetone = 0.791 g/mL
A student mixes 43.8 mL of acetone (58.08 g/mol, 0.791 g/mL)
43.8 mL = 43.8mL × 0.791g/mL
= 34.6458g ≈34.65g
1 mole of acetone = 58.08g
∴34.65g = 34.65g/58.08g
= 0.60mol
molecular weight of the product 1,5-diphenylpenta-1,4-dien-3-one = 234.29 g/mol
mole = mass/ molar weight
mole = 79.4g/ 234.29g/mol
mole(n) = 0.3389mol ≈ 0.34mol
1 mole of acetone will produce 1 mole of the product
∴0.60mol of acetone will produce 0.60mol of the product
but we get 0.34mol of the product
∴ % yield of the student's experiment is
\(\frac{0.34}{0.60}\) ˣ 100 = 56.67%
When does a chemical bond form? Explain in terms of attractions, repulsions and potential energy.
Answers
Answer:
A chemical bond forms when there is a net attractive force between two or more atoms due to the interaction of their electrons. This attractive force results from the balancing of two opposing forces: electron-electron repulsions and electron-nucleus attractions.
Explanation:
The repulsion between electrons arises from their negatively charged nature, which results in a repulsive force when two electrons are close together. On the other hand, the attraction between electrons and nuclei is due to the positively charged nuclei attracting the negatively charged electrons.
When the attractive force between electrons and nuclei overcomes the repulsive force between electrons, the energy of the system decreases, and a chemical bond is formed. This decrease in energy is reflected in a lower potential energy of the system, indicating that the bond is more stable than the individual atoms.
Therefore, the formation of a chemical bond can be understood as the result of an attractive force between the electrons and nuclei of the atoms, which leads to a decrease in potential energy and a more stable, lower-energy state.
complite the following reactions. NaOH(aq)+FeBr3(aq)→
Answers
Answer:
3NaOH+FeBr3>3NaBr+
Fe(OH)3
Explanation:
After writing the equation it has to be balanced
A Review Constants
following
60 - 463)
oblem.
Part A
H2C=CH-CH2-CH2-CH2-CH3
Spell out the full name of the compound.
Submit
Request Answer
th
Part B
CH .
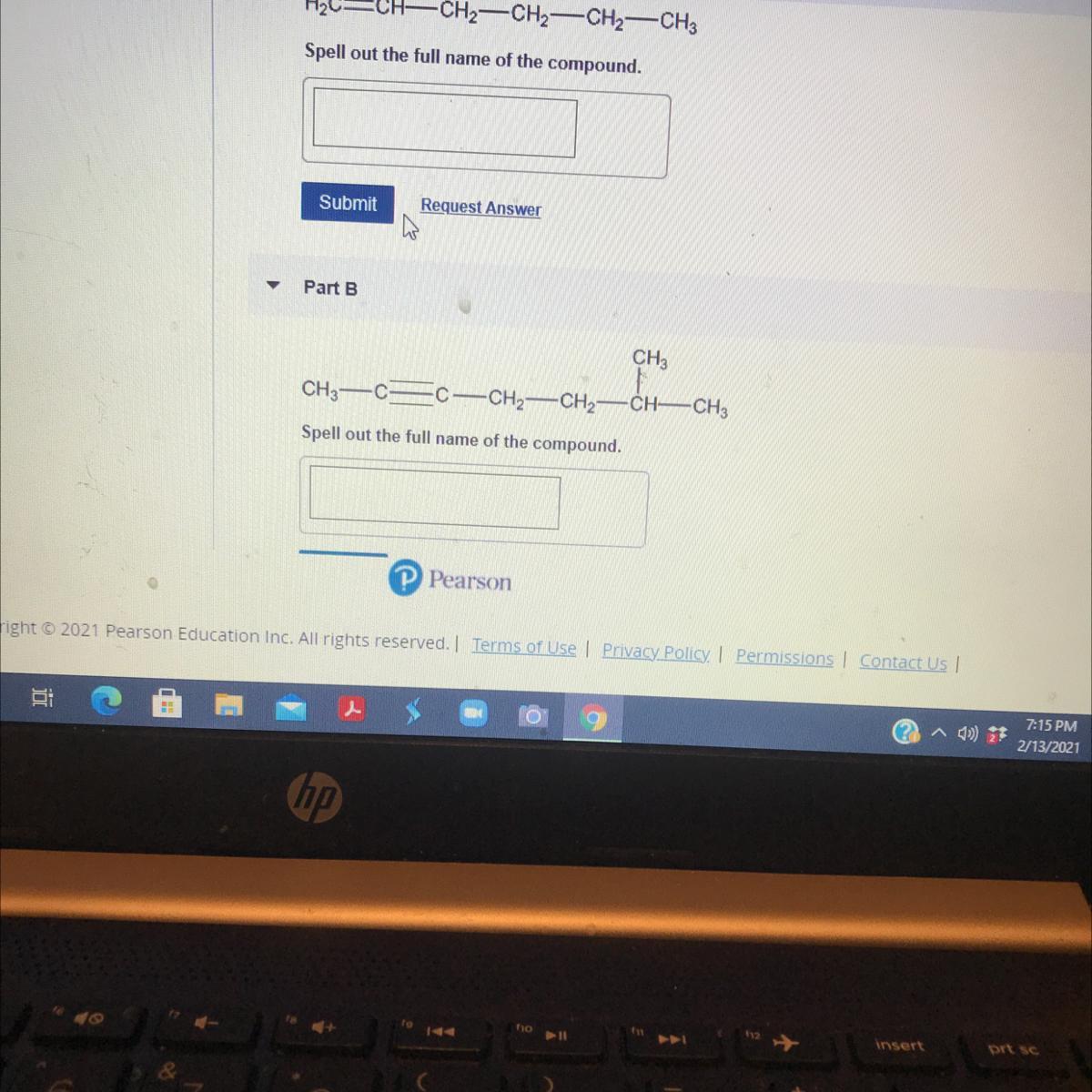
Answers
Answer:
A. 1–hexene
B. 6–methyl–2–heptyne
Explanation:
To name the compound given in the question above, we must obtain the following:
1. Determine the functional group of the compound.
2. Locate the longest continuous carbon chain. This gives the parent name the compound.
3. Identify the substituent group attached to the compound.
4. Locate the position of the substituent group by giving it the lowest possible count.
5. Combine the above to obtain the name of the compound.
Now, we shall determine the name of the compound.
A. Determination of the name of the compound.
1. The compound contains double bond (–C=C–). Hence, the compound is an alkene.
2. The longest continuous carbon chain is 6. Thus, the compound is hexane. But the presence of the double bond makes the compound to become hexene.
3. The functional group i.e double bond (–C=C–) is located at carbon 1.
4. The name of the compound is:
1–hexene
B. Determination of the name of the compound.
1. The compound contains triple bond (–C≡C–). Hence, the compound is an alkyne.
2. The longest continuous carbon chain is 7. Thus, the compound is heptane. But the presence of the triple bond makes the compound to become heptyne.
3. The substituent group attached to compound is –CH₃ i.e methyl.
4. The substituent group is located at carbon 6 and the functional group i.e triple bond (–C≡C–) is located at carbon 2.
NOTE: Numbering is done by giving the functional group the lowest possible count.
5. The name of the compound is:
6–methyl–2–heptyne
A Compound x consists of carbon 40%, hydrogen 6.7% and the rest being oxygen. If the relative molecular mass is Go, determine it's molecular formulas (C=12, H=1, 0=16) 3- Calculate the loss in mass when wog of calcium Carbonate is heated to a constant mass
Answers
From the calculation, the molecular mass of the compound would be C2H4O2
What is molecular formula?
We know that the molecular formula can be obtained from the use of the system;
Percentage of oxygen = 100 - (40 + 6.7)
= 53.3 %
C - 40/12, H - 6.7/1, O - 53.3/16
C - 3.33, H - 6.7, O - 3.33
Dividing through by the lowest ratio we have that
CH2O
We are told that the relative molecular mass of the compound is 60 then we have that;
(12 + 2 + 16)n = 60
n = 2
The molecular formula is;
C2H4O2
Learn more about molecular formula:https://brainly.com/question/12027614
#SPJ1
For the reaction
C+ O2 → CO2,
what is the maximum amount of CO2 which
could be formed from 3.67 mol of C and
4.23 mol of O₂?
Answer in units of g.
Answers
179.43 g/mol is the maximum amount of CO₂ which could be formed from 3.67 mol of C and 4.23 mol of O₂.
What is chemical reaction?
When molecules in a reactant undergo a chemical reaction, their bonds are broken, and molecules in the product undergo a similar process, new bonds are created, creating a new substance.
We are surrounded by chemical reactions on a daily basis, whether it be in our bodies as they process food or in the sun as they produce the light we see. Prior to starting with chemical reactions, it's crucial to understand physical and chemical changes.
C + O₂ → CO₂
carbon = 12.01g/mol
12.01 × 3.67
= 44.07 g/mol
O₂ = 32/mol
= 32 × 4.23
= 135.36 g/mol
44.07 g/mol + 135.36 g/mol
= 179.43 g/mol
Thus, 179.43 g/mol is the maximum amount of CO₂ which could be formed from 3.67 mol of C and 4.23 mol of O₂.
To know more about chemical reaction , click the link below ;
https://brainly.com/question/11231920
#SPJ1
Please answer this it’s 8th grade science and we are only 1 month into the year!

Answers
Answer:
Earth's gravitational pull
Explanation:
Astronauts in the space shuttle float because they are in “free fall” around Earth, just like a satellite or the Moon. Again, it is gravity that provides the centripetal force that keeps them in circular motion.
Cl2 + 2Na → 2NaCl How many grams of NaCl are produced by the reaction of 0.300 L of chlorine gas at STP with excess sodium?
Answers
Answer:
1.52 g of NaCl
Explanation:
We'll begin by calculating the number of mole of chlorine gas (Cl₂) that occupied 0.3 L at STP. This can be obtained as follow:
Recall: 1 mole of any gas occupy 22.4 L at STP.
1 mole of Cl₂ occupy 22.4 L at STP.
Therefore, Xmol of Cl₂ will occupy 0.3 L at STP i.e
Xmol of Cl₂ = 0.3 / 22.4
Xmol of Cl₂ = 0.013 mole
Thus, 0.013 mole of Cl₂ occupied 0.3 L at STP.
Next, we shall determine the number of mole of NaCl produced from the reaction.
Cl₂ + 2Na —> 2NaCl
From the balanced equation above,
1 mole of Cl₂ reacted to produce 2 moles of NaCl.
Therefore, 0.013 mole of Cl₂ will react to produce = 0.013 × 2 = 0.026 mole of NaCl.
Thus, 0.026 mole of NaCl is produced.
Finally, we shall determine the mass of the NaCl. This can be obtained as follow:
Mole of NaCl = 0.026 mol
Molar mass of NaCl = 23 + 35.5 = 58.5 g/mol
Mass of NaCl =?
Mole = mass /Molar mass
0.026 = mass of NaCl / 58.5
Cross multiply
Mass of NaCl = 0.026 × 58.5
Mass of NaCl = 1.52 g
Therefore, 1.52 g of NaCl were produced from the reaction
Are sperm and egg cells exact copies of the plant cell
Answers
Answer:
No
Explanation:
thats scientifically impossible
Could you guys please help me with this, I really don't have idea how to do?:(

Answers
The results of this investigation indicate that the quantity of salt dissolved in water affects how quickly an iron nail rusts.
What are the steps of the investigation of the rusting of nails?The steps of the investigation of the rusting of nails are as follows:
Introduction:
Rusting is a common process in which iron reacts with oxygen and water in the presence of an electrolyte to form hydrated iron (III) oxide, commonly known as rust. In this investigation, we will explore how the amount of salt dissolved in water affects the rusting reaction of an iron nail.
Materials:
Iron nail
Water
Salt
3 small beakers
Stopwatch
Paper towels
Procedure:
Fill each beaker with 50 ml of water.
Dissolve different amounts of salt in each beaker as follows:
Beaker 1: 0 grams of salt
Beaker 2: 5 grams of salt
Beaker 3: 10 grams of salt
Place an iron nail in each beaker.
Record the time and observe the nails every hour for 6 hours.
Record your observations and take photos of the nails at the end of each hour.
At the end of the experiment, dry the nails with paper towels and compare their appearance.
Observations:
Beaker 1: No visible rust on the nail throughout the experiment.
Beaker 2: A small amount of rust appeared on the nail after 2 hours. The rust increased over time and covered about 25% of the nail surface after 6 hours.
Beaker 3: A significant amount of rust appeared on the nail after 1 hour. The rust increased rapidly and covered about 80% of the nail surface after 6 hours.
Conclusion:
The results of this investigation suggest that the rusting reaction of an iron nail depends on the amount of salt dissolved in water. When no salt was added to the water, no visible rust appeared on the nail. However, when salt was added, rust appeared on the nail. The amount of rust increased with the amount of salt added, indicating that the rusting reaction is accelerated in the presence of an electrolyte such as salt. This is because the presence of ions in the solution helps to conduct electricity, which facilitates the transfer of electrons between the iron and oxygen molecules, thus accelerating the rusting process.
Learn more about rusting of iron at: https://brainly.com/question/29136931
#SPJ1
does food passes through liver, pancreas and appendix?
yes or no?
PLZ ANSWER ASAP PLZZZZZZZZZZZ
Answers
What was the niche of the lion king
Answers
Answer:
Known as the "king of the jungle" or the "king of beasts," lions are at the top of the food chain, and they hunt and eat other animals to survive. Their niche in the ecosystem allows them to help with other animal population control and prevent the spread of disease.
Explanation:
Observe: The Hollow pipe allows the water in each flask to move around and mix. Try several experiments with different temperatures in the top and bottom flasks.
Describe what you see
Answers
all of the following statements are true except some enzymes change shape and regular molecules either activators or inhibitors. enzymes are carbohydrates. enzymes are very sensitive
Answers
The invalid option is Enzymes are carbohydrates. Because the enzymes are generally proteins.
Enzymes are the protein molecules. Enzymes are the biological catalyst which can increases the rate of reaction by lowering the activation energy for the reaction by combining with the reagents but without undergoing any permanent chemical change.
Enzymes lower the activation energy necessary for the reaction to occur. This allows the reaction to happen more quickly than it would without an enzyme present.
The enzyme is not destroyed during the reaction and can be reused.
So, we can state that the wrong answer is enzymes are carbohydrates.
To learn more about Enzymes, Here :
https://brainly.com/question/13554219?referrer=searchResults
#SPJ4
Question 8
A certain cation has five atoms and an overall charge of +1. Which of the following combinations of
formal charges on the five atoms is indicative of the "best" Lewis structure?
Answers
Answer:
0, 0, 0, +1, 0
Explanation:
Because the compound has a +1 charge overall, the sum of the formal charges must equal +1. The correct answer is the Lewis structure with as many atoms as possible having the formal charge of 0 with just a single atoms having the +1 formal charge.
we can smell because we have nose
Answers
yes it's true.
we can smell cause we have nose have smelling sense.
Answer:
Really? I thought we had noses to poke in other people's business
A 3.00 L flexible container holds a sample of hydrogen gas at 153 kPa.
The pressure increases to 304 kPaand the temperature remains constant.
What will the volume be?
0.66 L
1.51 L
2.26 L
4.50 L
Answers
The volume of the flexible container that holds a sample of hydrogen gas at 153 kPa is 1.51L (option B).
How to calculate volume?The volume of a substance can be calculated using the following formula:
P1V1 = P2V2
Where;
P1 = initial pressureP2 = final pressureV1 = initial volumeV2 = final volume153 × 3 = 304 × V2
459 = 304V2
V2 = 459/304
V2 = 1.51L
Therefore, the volume of the flexible container that holds a sample of hydrogen gas at 153 kPa is 1.51L.
Learn more about volume at: https://brainly.com/question/2098026
#SPJ1
At 25 °C, only 0.0510 mol of the generic salt AB is soluble in 1.00 L of water.
What is the sp of the salt at 25 °C?
AB(s)↽−−⇀A+(aq)+B−(aq)
Answers
The value of the solubility product constant (Ksp) for the salt AB at 25°C is 2.60 x 10⁻³.
The solubility product constant (Ksp) is the equilibrium constant for the dissolution of a sparingly soluble salt in water. It is given by the expression Ksp = [A⁺][B⁻] where [A⁺] and [B⁻] are the molar concentrations of the cations and anions in solution, respectively.
In this case, the balanced equation for the dissolution of the salt AB is: AB(s) ⇌ A⁺(aq) + B⁻(aq) We know that at 25°C, only 0.0510 mol of the salt AB is soluble in 1.00 L of water. This corresponds to a molar solubility of
s = 0.0510 mol / 1.00 L = 0.0510 M
At equilibrium, the molar concentration of A⁺ and B⁻ will also be 0.0510 M. Therefore, the value of Ksp for the salt AB at 25°C can be calculated as: Ksp = [A⁺][B⁻] = (0.0510 M) * (0.0510 M) = 2.60 x 10⁻³.
To know more about solubility product:
https://brainly.com/question/30186409
#SPJ1
Heart, 5 stars, and Brainiest if right! Answer needed ASAP PLEASE!What type of forces do not change the motion of an object?
A. Acceleration forces
B. Balanced forces
C. Inertia forces
D. Unbalanced forces
Answers
Answer:
unbalanced force
Explanation:
pls mark me brainliest
Answer:
B. balanced forces
Explanation:
the motion of an object will not change if the forces pushing or pulling the object are balanced
What would have happened to your % Transmittance reading and to your calculations of Keq if the spectrophotometer had been set at 520 nm rather than 447nm
Answers
Answer:
On the off chance that the wavelength(λ) maximum worth has been changed to 520 nm from 470 nm on the spectrophotometer, less light would be absorbed and in this way %T would be higher than the one found at 470 nm. The wavelength utilized at 520 nm isn't adequate for the excitation and consequently lesser light is absorbed by the arrangement.
Explanation:
A spectrophotometer is an analytical equipment used to quantitatively gauge the transmission(passage) or impression of visible light, UV light or infrared light through a medium.
Transmittance (τ) is the ratio of the brilliant or luminous flux at a given wavelength that is transmitted to that of the incident radiation.
where, Keq is the equilibrium constant.
On the off chance that the wavelength(λ) has been changed to 520 nm from 470 nm on the spectrophotometer, less light would be absorbed and in this way %T would be higher than the one found at 470 nm.
What happens to Transmittance?A spectrophotometer is an analytical equipment used to quantitatively gauge the transmission(passage) or impression of visible light, UV light or infrared light through a medium. Transmittance (τ) is the ratio of the brilliant or luminous flux at a given wavelength that is transmitted to that of the incident radiation. The wavelength utilized at 520 nm isn't adequate for the excitation and consequently lesser light is absorbed by the arrangement. As the concentration goes up, more radiation is absorbed and the absorbance goes up. Therefore, the absorbance is directly proportional to the concentration.
Find more information about Transmittance here: brainly.com/question/14919298
Oxygen has a boiling point of -183°C and freezing point of -219°C which of the following is true about oxygen?
A. It turns into a solid at the same temperature as water.
B. It boils at a higher temperature than water boils.
C. It cannot be found naturally as a solid on Earth.
D. It cannot be found naturally as a gas on Earth
Answers
Answer:
B. It boils at a higher temperature than water boils.
Explanation:
The correct answer is B. Oxygen boils at a higher temperature than water boils.
Water has a boiling point of 100°C and a freezing point of 0°C, so it turns into a solid at a higher temperature than oxygen.
Oxygen can be found naturally as a solid on Earth, such as in the form of dry ice (solid carbon dioxide) or in certain minerals. However, it is more commonly found as a gas in the Earth's atmosphere.
how much energy is required to heat 500g of ice at 0⁰C to 60⁰C?
a) 125,400 J
b) 167,000 J
c) 292,400 J
d) 41,883,600 J
Answers
The amount of energy needed to heat 500 g of ice at 0⁰C to 60⁰C is 292,400 J. Option C.
Energy of reactionIn order to calculate the energy required to heat the ice, we need to consider two stages: first, we need to calculate the energy required to melt the ice, and second, we need to calculate the energy required to heat the resulting liquid water to 60°C.
To melt the ice, we need to supply energy equal to the heat of fusion of ice. The heat of fusion of ice is 334 J/g. Therefore, the energy required to melt 500 g of ice is:
Q1 = (334 J/g) x (500 g) = 167,000 J
Once the ice is melted, we need to heat the resulting liquid water to 60°C. The specific heat capacity of water is 4.184 J/(g°C). Therefore, the energy required to heat 500 g of water from 0°C to 60°C is:
Q2 = (4.184 J/(g°C)) x (500 g) x (60°C - 0°C) = 125,520 J
The total energy required to melt the ice and heat the resulting liquid water to 60°C is the sum of Q1 and Q2:
Q = Q1 + Q2 = 167,000 J + 125,520 J = 292,520 J
Thus, the amount of energy needed to heat 500 g of ice at 0⁰C to 60⁰C is 292,400 J.
More on energy of reactions can be found here: https://brainly.com/question/1865119
#SPJ1
3. The speed of a reaction can be increased by increasing reactant concentration or decreasing
particle size. *
(1 Point)
True
False
Answers
Answer:
true
because with the both states we increase the surface of reaction
When 7.59 grams of sodium hydroxide (NaOH) are dissolved in 80.0 grams of water at 25.0 °C in an insulated container, the temperature of the water increases to 48.0 °C. Assuming that the specific heat of the solution is 4.184 J/(g °C) and that no heat is gained or lost by the container, what is the ∆H of solution of NaOH in kJ/mol?
Answers
The ∆H of solution of NaOH is 46.8 kJ/mol.
First, we need to calculate the amount of heat absorbed by the solution:
q = m × c × ∆T
where q is the heat absorbed (in Joules), m is the mass of the solution (in grams), c is the specific heat capacity of the solution (in J/(g °C)), and ∆T is the change in temperature (in °C).
In this case, the mass of the solution is the sum of the mass of NaOH and the mass of water:
m = 7.59 g + 80.0 g = 87.59 g
The change in temperature is:
∆T = 48.0 °C - 25.0 °C = 23.0 °C
Substituting the values, we get:
q = 87.59 g × 4.184 J/(g °C) × 23.0 °C = 8,878 J
Next, we need to convert the heat absorbed into the enthalpy change of solution (∆H). The enthalpy change of solution is the heat absorbed per mole of solute. The number of moles of NaOH is:
n = m/M
where M is the molar mass of NaOH, which is 40.00 g/mol.
n = 7.59 g / 40.00 g/mol = 0.1898 mol
Therefore, the enthalpy change of solution is:
∆H = q/n = 8,878 J / 0.1898 mol = 46,780 J/mol = 46.78 kJ/mol
The H of a NaOH solution, rounded to three significant numbers, is 46.8 kJ/mol.
To know more about the Temperature, here
https://brainly.com/question/30411639
#SPJ1
A solution with a pH of 8 is?
Answers
Answer:base
Explanation:
base
A solution with a pH of 8 is basic in nature.
pH is defined as the negative logarithm of H⁺ ion concentration.
pH is a measure of how acidic or basic a substance is. In our everyday routine, we encounter and drink many liquids with different pH. Water is a neutral substance. Soda and coffee are often acidic.
The pH is an important property, since it affects how substances interact with one another and with our bodies. In our lakes and oceans, pH determines what creatures are able to survive in the water.
Learn more about pH, here:
https://brainly.com/question/2288405
#SPJ1
How much heat will be absorbed by 122 g of water when its temperature is raised by 17oC? The specific heat for water is 4.18 J/g oC. Use significant figures.
Answers
Answer:
Heat energy = 8669.32J
Explanation:
Mass = 122g
Change in temperature ∇T = 17°C
Specific heat capacity (c) = 4.18J/g°C
Heat energy (Q) = ?
Heat energy (Q) = mc∇T
Q = heat energy
M = mass of the substance
C = specific heat capacity of the substance
∇T = change in temperature of the substance
Q = mc∇T
Q = 122 × 4.18 × 17
Q = 8669.32J
The heat energy required to raise 122g of water by 17°C is 8669.32J
Answer:
\(Q=8669.3J=8.67kJ\)
Explanation:
Hello,
In this case, we can compute the absorbed heat of water needed to raise its temperature by 17 °C by using the following formula:
\(Q=mCp\Delta T\)
In this case, the mass is 122 g, the specific heat 4.18 J/(g°C) and the temperature change 17 °C, hence the heat turns out:
\(Q=122g*4.18\frac{J}{g\°C}*17\°C\\ \\Q=8669.3J=8.67kJ\)
Best regards.
1) To increase the amount of NH3 at 200 atm, the manufacturer should (increase, decrease, not change) the temperature of the reaction chamber.
2) This change in temperature would shift the reaction to the (left, right) because this equilibrium reaction is (exothermic, endothermic)
Answers
The temperature of the reaction should be decreased
This change in temperature would make the equilibrium to shift to the right.
What is the LeChatelier principle?
The Le Chatelier's principle, commonly referred to as the Le Chatelier's principle of equilibrium, is a chemical principle that describes how an equilibrium system reacts to environmental changes.
According to this theory, when an equilibrium system is exposed to an outside force, it will respond in a way that partially offsets the imposed change and restore equilibrium.
Learn more about LeChatelier principle:https://brainly.com/question/31377984
#SPJ1
Which equations represent inverse variation? Check all that apply.
O y = 2x
pu = 13
z = 2
X
4 =
h = 99
1= ⁹0
Answers
Answer:
y = 2x pv = 13 z = (2/x) 4 = (y/x) h = (9g/5) Inverse variation is represented by the equation y = k/x, where k is a constant.
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
An error during which cellular process would create a gene mutation?
Answers
An error during DNA replication would create a gene mutation.
During DNA replication, the genetic information in a cell is copied to make new DNA molecules. However, mistakes can occur during this process, leading to changes in the DNA sequence, which can result in a mutation. Mutations can also be caused by exposure to environmental factors, such as radiation or chemicals, which can damage the DNA molecule directly or affect the cellular processes involved in DNA replication.
Mutations can have a variety of effects on the organism, ranging from no effect to causing serious health problems or even death. Gene mutations can also be inherited from a parent, which can result in genetic disorders or predisposition to certain diseases. Therefore, it is important to understand the mechanisms of gene mutations and their potential impacts on organisms.
To know more about the Gene mutation, here
https://brainly.com/question/15448555
#SPJ1