Complete and balance the following redox reaction under acidic conditions. When properly balanced using the smallest whole number coefficients, the coefficient of S is H2S (g) + NO3-(aq) -> S (s) + NO(g)
Answers
The balanced redox reaction is as follows:H2S(g) + NO3-(aq) → S(s) + NO(g)Step-by-step solution:Redox reactions are those reactions that involve both oxidation and reduction simultaneously.
Such reactions are balanced using the half-reaction method. Let's balance the given redox reaction using the half-reaction method:Half-reaction of oxidation:H2S → SIn this reaction, hydrogen is oxidized to form Sulfur. The oxidation state of hydrogen changes from +1 to 0 and the oxidation state of Sulfur changes from -2 to 0. So, two electrons must be added to the left side of the equation.H2S → S + 2e- .... (1)Half-reaction of reduction:NO3- → NOIn this reaction, nitrogen is reduced to form nitric oxide.
The oxidation state of Nitrogen changes from +5 to +2 and the oxidation state of oxygen changes from -2 to 0. So, 3 electrons must be added to the right side of the equation.NO3- + 3e- → NO ..... (2)After balancing the two half-reactions, they should be added to obtain the complete balanced equation.(i) Multiply equation (1) by 2 to balance electrons.2H2S → 2S + 4e- .... (3)(ii) Add equation (3) and equation (2) to obtain the complete balanced equation.2H2S + NO3- + 3H+ → 2S + NO + 4H2O .... (4)Thus, the balanced redox reaction is H2S (g) + NO3-(aq) → S (s) + NO(g) with the smallest whole number coefficients and the coefficient of S is 1.
To know more about reaction visit:
https://brainly.com/question/30464598
#SPJ11
Related Questions
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
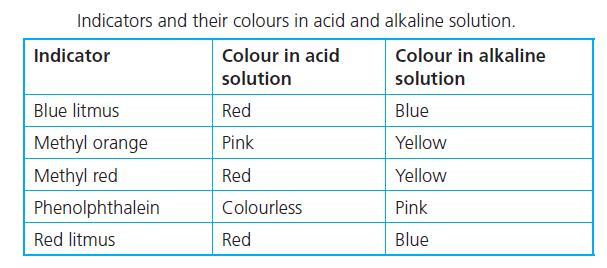
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
Which type of variable is kept the same throughout an experiment?
ILL GIVE BRAINLYIST
Answers
Answer:
A control variable
Explanation:
Answer:
Control variable
Explanation:
Substance A has a heat capacity that is much greater than that of substance B. If 10.0 g of substance A initially at 30.0 ∘C is brought into thermal contact with 10.0 g of B initially at 70.0 ∘C, what can you conclude about the final temperature of the two substances once the exchange of heat between the substances is complete?
Answers
Answer:
E دی کر دیکھ زور شکر 75 سلام والے آج داد ہی auditory آپ لالچ زچ فنڈنگ ایف دن
The first-order decomposition of cyclopropane has a rate constant of 6. 7 x 10^-4 s-1. If the initial concentration of cyclopropane is 1. 33 m, what is the concentration of cyclopropane after 644 s?.
Answers
The concentration of cyclopropane after 644 s can be calculated using the first-order decomposition rate constant and the initial concentration of cyclopropane.
The concentration of cyclopropane after a certain time can be determined using the following formula: [Cyclopropane]t = [Cyclopropane]0 e^(-kt), where [Cyclopropane]t is the concentration of cyclopropane after time t, [Cyclopropane]0 is the initial concentration of cyclopropane, k is the rate constant, and e is the mathematical constant.
Plugging in the given values, we get [Cyclopropane]t = 1.33 e^(-6.7x10^-4x644) = 0.725 M. Therefore, the concentration of cyclopropane after 644 s is 0.725 M.
The question involves first-order kinetics, which is a type of chemical reaction where the rate of the reaction is proportional to the concentration of the reactant. In this case, the reaction is the decomposition of cyclopropane. The rate constant (k) is a proportionality constant that relates the rate of the reaction to the concentration of the reactant.
The formula [Cyclopropane]t = [Cyclopropane]0 e^(-kt) is derived from the first-order rate law, which states that the rate of the reaction is proportional to the concentration of the reactant raised to the power of the order of the reaction. In this case, the order of the reaction is 1 because it is a first-order reaction.
The mathematical constant e is used in the formula because it represents the natural exponential function, which describes the behavior of many natural phenomena, including chemical reactions. By plugging in the given values and solving for the concentration of cyclopropane after 644 s, we can determine the extent of the reaction at that time.
To know more about cyclopropane refer to
https://brainly.com/question/18521496
#SPJ11
Numbering the Steps for Balancing Equations Number the steps for balancing equations: Use coefficients to increase the atoms on each side. Check to make sure you have the same number of each type of atom on each side. Count the atoms on each side. Identify the atoms on each side. Intro Done
Answers
In order to balance a chemical equation steps to be followed are : Identify atoms on each side.Count atoms on each side.Numbering steps for balancing Equations in sequential order.Use coefficients to increase atoms on each side.Check to make sure you have same number of each type of atom on each side.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
Learn more about chemical equation,here:
https://brainly.com/question/30087623
#SPJ9
Your question is is incomplete, the complete question will be:
Numbering the Steps for Balancing Equations in a sequential order:
Use coefficients to increase the atoms on each side.
Check to make sure you have the same number of each type of atom on each side.
Count the atoms on each side.
Identify the atoms on each side.
What is the name of the compound H₃S₅?
Answers
9. During a titration, 50.0 ml of 0.2M NaOH were required to neutralize 50.0ml of H_{3}*P * O_{4} What's the concentration of the H_{3}*P * O_{4} solution?
Answers
Answer:
0.067M H3PO4
Explanation:
H3PO4 reacts with NaOH as follows:
H3PO4 + 3NaOH → 3H2O + Na3PO4
Where 1 mole of H3PO4 reacts with 3 moles of NaOH
To solve trhis question we need to find the moles of NaOH required. With the chemical equation we can find the moles of H3PO4 and its concentration as follows:
Moles NaOH:
50.0mL = 0.0500L * (0.20moles /L) = 0.0100 moles NaOH
Moles H3PO4:
0.0100 moles NaOH * (1mol H3PO4 / 3mol NaOH) = 0.00333 moles H3PO4
Concentration:
0.00333 moles H3PO4 / 0.0500L = 0.067M H3PO4
if dfb = 3 and dft = 29 and fobt = 3.15, what would you conclude using a = 0.05?
Answers
if dfb = 3 and dft = 29 and fobt = 3.15, using the given we can can reject our null hypothesis, H_0.
Option (a) is the correct choice.
From the available information,
d fB=3, d fT=29, alpha=0.05, F_{obt}=3.15
Then, dfw = dfT-dfB = 29-3 = 26
In variance analysis, the F value is utilized (ANOVA). Two mean squares are divided to calculate it.
The ratio of explained variance to unexplained variance is calculated using this formula. A theoretical distribution is the F distribution.
In hypothesis testing, F Statistics are looked up using the F Table. Although it's more typical to perform tests using software like Excel or SPSS, the F Table might be helpful for rapidly searching up several data at once.
From the F-table,
The tabulated value at F_{0.05}(3,26) is 2.975.
Here the calculated value 3.15 is greater than the tabulated value 2.975,
So, we can reject our null hypothesis, H_0.
For more questions on null hypothesis
https://brainly.com/question/4436370
#SPJ4
The complete question should be like:
if dfb = 3 and dft = 29 and fobt = 3.15, what would you conclude using a = 0.05?
(a) reject H_0
(b) reject H_1
(c) retain H_0
(d) retain H_1

pls balance the following chemical equation asap; Ca(OH)2 +CO2 = CaCO3+ H2O pls make it step by step
Answers
Answer:
Explanation:
Start by writing water as HOH
Ca(OH)2 +CO2 = CaCO3+ HOH
Next pay attention to the CO2 going to CO3
We need an oxygen.
Fortunately that is provided by the (OH)2
Now we have
Ca(OH)2 + CO2 ==> CaCO3 + HOH
and believe it or not, that is balanced as it is
The left side has 1 Ca. So does the right side
The Left side has 1 C. So does the right side.
The left side has 2 H. So does the right side
The left side has 2 oxygens (in Ca(OH2)) + 2 oxygens in CO2
So the equation is balanced.
Why Does Hot Coco Have a Higher Temperature Then Cold Milk? Explain In terms of Molecules and energy.
hi
Answers
Answer:
When you pour cold milk into hot cocoa, the milk and cocoa particles start to collide When a high-energy cocoa particle hits a low-energy milk particle, energy transfers. The cocoa particles slow down, and the cup of cocoa cools down. ... Lower kinetic energy means lower temperature.
Explanation:
hope this helps a little have a good night :) ❤
Why does alcohol evaporate faster than water? *
1.Alcohol evaporates faster than water because alcohol molecules are hotter
2.Alcohol evaporates faster than water because alcohol molecules are not as attracted
to each other as water molecules are
3.Alcohol evaporates faster than water because alcohol molecules are more attracted
to each other than water molecules are (Which one I need help)
Answers
Answer:
Number oneExplanation:
Alcohol has a lower boiling point than water. Water boils at 100 degrees Celsius (212 degrees Fahrenheit), while Alcohol boils at 82 degrees Celsius (179.6 degrees Fahrenheit).which alkyl halide would form the most stable carbocation: isopropyl bromide, tert-butyl bromide, methyl bromide or ethyl bromid
Answers
T-butyl bromide alkyl halide would result in the formation of the most stable carbocation. The quantity of methyl groups bonded to the core carbon is often associated with carbocation stability.
A halide is what in chemistry?Halogens are a class of chemical compounds known as halides. There are halides in the natural world, and some of them, like salts and acids, are essential to sustaining life. Halides can be found in minerals, organisms, and plants. NaCl, or table salt, is the most widely used halide.
Why does halide group exist?The class of minerals known as halides includes the sodium, salt, and salts of hydrochloric acid. The crystals halite, sylvite, and carnallite are part of this group of minerals, and they exclusively include chloride that has petrogenic significance. Halite, another name for rock salt (NaCl).
To know more about Halide visit:
brainly.com/question/28384269
#SPJ4
when iron-rich materials cool below their __________ temperature, they may preserve the orientation of the magnetic field
Answers
When iron-rich materials cool below their Curie temperature, they may preserve the orientation of the magnetic field.
The Curie temperature is the temperature at which a ferromagnetic material loses its magnetic properties. When a ferromagnetic material is heated to its Curie temperature, the thermal energy causes the magnetic moments of the atoms in the material to become disordered, which causes the material to lose its magnetic field. When the material cools below its Curie temperature, the magnetic moments of the atoms become ordered again, but the orientation of the magnetic field may not be preserved. However, in iron-rich materials, if the cooling process is slow enough, the orientation of the magnetic field can be preserved, creating a record of the Earth's magnetic field at the time the material cooled. This property has been used to study the Earth's magnetic field history by analyzing the magnetic properties of rocks and sediments.
To learn more about ferromagnetic material click here : brainly.com/question/13399259
#SPJ11
. Which of the following statements about solutions is false?
a. All solutions are mixtures.
b. All solutions are homogeneous.
c. All solutions contain two or more compounds.
d. All solutions contain at least two substances.
Answers
Answer:
B
Explanation:
The statement false about solution has been that all solutions are mixtures. Thus, option A is correct.
The solution can be defined as the dissolution of two or more substances. Thus, all solutions are been considered as dissolution of the solute and solvent molecules.
The solution has generally been the homogenous mixture as there has been the complete dissolution of the substances. However, the mixture can be defined as the combination and not the complete dissolution of the substances.
Thus, all solutions have not been considered as mixtures. Thus, option A is correct.
For more information about the solutions, refer to the link:
https://brainly.com/question/7932885
Which description best characterizes the motion of particles in a solid?
slow but able to move past one another
fast and widely spaced
not moving
vibrating around fixed positions
Answers
Answer:
d
Explanation:
Answer:
D
Explanation:
IM JUST THAT DUDE
Bacteria and archaea are examples
of ...
Answers
Answer:
prokaryotes
Explanation:
How many electrons would be in a calcium cation with a positive 2 charge?
Answers
Number of electrons in a Ca²⁺ = 18
Further explanationIn an atom there are levels of energy in the shell and sub shell
This energy level is expressed in the form of electron configurations.
Writing electron configurations starts from the lowest to the highest sub-shell energy level. There are 4 sub-shells in the shell of an atom, namely s, p, d and f. The maximum number of electrons for each sub shell is
• s: 2 electrons
• p: 6 electrons
• d: 10 electrons and
• f: 14 electrons
Charging electrons in the sub shell uses the following sequence:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶, 5s², 4d¹⁰, 5p⁶, 6s², etc.
The element Ca has an atomic number of 20, so the number of electrons and protons (in neutral atoms) is also 20
Electron configuration of Ca : 1s², 2s², 2p⁶, 3s², 3p⁶, 4s² ⇒[Ar] 4s²
When Ca releases 2 electrons to gain stability (forming Ca²⁺ cations), the number of electrons becomes:
\(\tt 20-2 = 18\)
and the electron configuration (Ca²⁺ ) becomes:
1s², 2s², 2p⁶, 3s², 3p⁶
Consider the following three-step mechanism for a reaction: Cl2 (g) ⇌ 2 Cl (g) Fast Cl (g) CHCl3 (g) → HCl (g) CCl3 (g) Slow Cl (g) CCl3 (g) → CCl4 (g) Fast Identify the intermediates in the mechanism.
Answers
The intermediates in the given three-step mechanism are Cl (g) and CCl3 (g).
In the mechanism, Cl2 (g) is in equilibrium with 2 Cl (g), indicating that Cl (g) is an intermediate formed during the reaction. This means that Cl2 (g) breaks apart into Cl (g) molecules, which then go on to react with other species in subsequent steps.
In the second step, Cl (g) reacts with CHCl3 (g) to form HCl (g) and CCl3 (g). Here, Cl (g) is consumed as it reacts with CHCl3 (g) to produce the products.
In the third step, Cl (g) reacts with CCl3 (g) to form CCl4 (g). This step consumes Cl (g) as it reacts with CCl3 (g) to produce the final product.
Overall, the intermediates in this three-step mechanism are Cl (g) and CCl3 (g). They are formed in intermediate steps of the reaction and are consumed in subsequent steps to yield the final products.
To learn more about equilibrium click here : brainly.com/question/30694482
#SPJ11
How much potassium nitrate could be dissolved into 2 L of water
Answers
Answer:
640 grams
Explanation:
look up Solubility table in wikipedia for potassium nitrate (KNO3)
32 grams of potassium nitrate (KNO3) water solubility at 20 degrees celsius (room temperature) can be dissolved in 100 milliliters (0.1 L) of water.
2 liters = 2000 milliliters
32 grams / 100 milliliters = x grams / 2000 milliliters
cross-multiply
100 * x = 32 * 2000
x = (32 * 2000) / 100
x ≈ 640 grams
chatgpt
Scientit ue electricity to break down water and hydrogen ga and oxygen ga which tatement bet explain what happen
Answers
The water molecules are split into hydrogen and oxygen gases by the electric current. Then, the gases of hydrogen and oxygen are released into the environment. Electrolysis is the name of this procedure.
The water molecules are disassembled by electrolysis using electricity into their component elements of hydrogen and oxygen, which are subsequently liberated as gases.
A procedure called electrolysis employs electric current to separate a substance into its constituent parts. In electrolysis, an electric current is used to drive ions in a solution towards electrodes, where they combine to generate a new molecule. The method is generally used to refine metals and other chemicals as well as to extract metals from their ores.
For more Questions on electrolysis click on
https://brainly.com/question/12994141
#SPJ4
what questions should I expect on the Chemistry module 4 DBA?
(When I’m finished with the DBA I’ll answer this question but right now I need help)
Answers
Decomposition processes should appear here on Chemistry previous modules DBA, When I'm done with the DBA, I'll respond to this query.
How do you define reaction?Opposition or antagonism to a force, effect, or movement is a reactionary act, process, or occurrence. especially: a reaction to a particular treatment, circumstance, or stimulus; leaning toward a past and typically antiquated political or social system or policy.
What kinds of reactions are there?Combination, disintegration, single-replacement, twofold, and combustion are the five fundamental types of chemical reactions. You can classify a reaction into one of these groups by looking at the reactants and products.
To know more about reactions visit:
https://brainly.com/question/28984750
#SPJ1
Answer: I was asked to classify equations, balance equations, and define + explain the law of conservation of mass
Calculate the pH for each of the cases in the titration of 35.0 mL of 0.180 M KOH(aq) with 0.180 M HI(aq). Note: Enter your answers with two decimal places.
Answers
The pH at the equivalence point is 7.00, before the equivalence point is 0.74 (basic), and after the equivalence point is 0.74 (acidic).
In this titration, we have a strong base (KOH) reacting with a strong acid (HI). At the equivalence point, all the KOH will have reacted with HI to form KI and H₂O. We can use the stoichiometry of this reaction to calculate the number of moles of HI needed to reach the equivalence point.
First, we need to determine the volume of HI needed to reach the equivalence point. Since we have 35.0 mL of 0.180 M KOH, we can use the equation M1V1 = M2V2 to find the number of moles of KOH present:
0.180 M x 0.0350 L = 0.00630 mol KOH
Since the reaction between KOH and HI is 1:1, we need 0.00630 moles of HI to reach the equivalence point. Using the same equation, we can find the volume of HI needed:
0.180 M x V(HI) = 0.00630 mol HI
V(HI) = 0.0350 L
At the equivalence point, the solution will contain only KI and water. The pH of this solution will be neutral, or 7.00.
Before the equivalence point, the KOH is in excess and the solution is basic. We can use the equation for the reaction of KOH and water to calculate the concentration of hydroxide ions:
KOH(aq) + H₂O(l) → K⁺(aq) + OH⁻(aq)
The initial concentration of KOH is 0.180 M, so the concentration of OH⁻ will also be 0.180 M. Using the equation for the ion product constant of water, we can calculate the pH:
pH = -log[OH⁻] = -log(0.180) = 0.74
After the equivalence point, the HI is in excess and the solution is acidic. We can use the equation for the reaction of HI and water to calculate the concentration of hydronium ions:
HI(aq) + H₂O(l) → H₃O⁺(aq) + I⁻(aq)
The initial concentration of HI is 0.180 M, so the concentration of H₃O⁺ will also be 0.180 M. Using the equation for pH, we can calculate the pH:
pH = -log[H₃O⁺] = -log(0.180) = 0.74
Therefore, the pH at the equivalence point is 7.00, before the equivalence point is 0.74 (basic), and after the equivalence point is 0.74 (acidic).
To know more about pH, refer to the link below:
https://brainly.com/question/31132561#
#SPJ11
2. Consider dimethyl ether at 300 K which has an angle averaged radius of 0.25 nm. a) Calculate its collision frequency at 1 bar and 1 Pa. b) Calculate its decomposition rate constant k (CH3)2CO produ
Answers
a) The collision frequency of dimethyl ether can be calculated using the kinetic theory of gases. The collision frequency is given by the equation:
\(\[\text{{Collision frequency}} = \frac{1}{4} \sqrt{\frac{8 \cdot k \cdot T}{\pi \cdot m}}\]\)
where k is the Boltzmann constant, T is the temperature in Kelvin, and m is the mass of dimethyl ether molecule. Given that the angle-averaged radius of dimethyl ether is 0.25 nm, we can calculate the mass of the molecule using its density or molar mass.
b) To calculate the decomposition rate constant of (CH3)2CO, we need additional information such as the reaction mechanism and reaction conditions. The rate constant for a chemical reaction depends on factors like temperature, activation energy, and the presence of catalysts. Without these details, it is not possible to calculate the decomposition rate constant accurately.
In conclusion, the collision frequency of dimethyl ether at a specific temperature can be calculated using the kinetic theory of gases. However, to calculate the decomposition rate constant of (CH3)2CO, additional information about the reaction conditions and mechanism is needed.
To know more about Molar Mass visit-
brainly.com/question/31545539
#SPJ11
pls help, I will give Brainlist if you answer correct. pls

Answers
Explanation:
The union of vinegar and bicarbonate produces carbon dioxide
The carbonic acid, which is weaker, in turn breaks down into water and carbon dioxide, which being volatile separates
will observe a yellow color, confirming that BASIC HYDROLYSIS has taken place. To the touch the bottle cools and in the end a white deposit may remain on the bottom.
Joshua has a mixture of sand and iron fillings which physical property would best help Joshua separate the fillings from the sand?
Answers
Answer:
Magnetism
Explanation:
The iron fillings would be magnetic therefore using a magnet would be the simplest way to separate the two.
how many flourine atoms are in 410 g of UF6
Answers
3.6 ×10²⁴ atoms fluorine are in 410 g of UF\(_6\). Fluorine is an atomic number 9 chemical element with both the symbol F.
What is fluorine?Fluorine is an atomic number 9 chemical element with both the symbol F. This is the smallest halogen as well as occurs as a very poisonous, pale yellow diatomic vapor under normal circumstances.
It is exceptionally reactive being the most electronegative active catalyst, reacting with all other elements save the light inert.
mole = 410 / 352.02 =1.16mole
number of atom= 1.16× 6.022×10²³=6.98×10²³
number of atom of fluorine =6× 6.98×10²³= 3.6 ×10²⁴ atoms
Therefore, 3.6 ×10²⁴ atoms fluorine are in 410 g of UF\(_6\).
To learn more about fluorine, here:
https://brainly.com/question/10700214
#SPJ1
A study was conducted of 90 adult male patients following a new treatment for congestive heart failure. One of the variables measured on the patients was the increase in exercise capacity (in minutes) over a 4-week treatment period. The previous treatment regime had produced an average increase of μ=2 minutes. The researchers wanted to evaluate whether the new treatment had increased the value of μ in comparison to the previous treatment. The data yielded y(bar)=2.17 and s=1.05.
(a) if the actual value of mu is 2.1 and alpha is reduced from 0.05 to 0.01, what would be the effect on the power curve?
(b) If the sample size is reduced from 90 to 50, what would be the effect on the power curve?
Answers
a. Decreasing alpha from 0.05 to 0.01 makes the significance level more stringent. You will be less likely to reject the null hypothesis, even when it's false. This increases the probability of a Type II error, thus potentially reducing the power of the test. The power curve will shift to the left.
b. If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
What more should you know about decreasing the alpha and the power curve?The power curve is a graph that shows the probability of rejecting the null hypothesis as a function of the true value of the mean.
In the given scenarios of this study, Reducing the significance level and reducing the sample size will shift the power curve to the left, indicating a decrease in the statistical power of the test.
The power of a statistical test is the probability that it correctly rejects the null hypothesis when the alternative hypothesis is true.
a) Reducing alpha from 0.05 to 0.01 means that we are more stringent in our assessment of whether the new treatment is effective.
This will result in a decrease in the power of the test, meaning that it is less likely that we will be able to detect a difference between the new treatment and the previous treatment.
b) If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
This is because a smaller sample size decreases the power of the test. A larger sample size provides more information and thus makes it more likely to correctly reject the null hypothesis when the alternative hypothesis is true.
Therefore, by reducing the sample size, you are decreasing the likelihood of detecting a true effect if one exists, thus reducing the power of the test.
Find more exercises on alpha level in a study;
https://brainly.com/question/6372035
#SPJ4
HELP ASAP WILL MARK BRAINLIEST!!!

Answers
Answer:
22.4/3.8=5.89
Explanation:
Under which conditions of temperature and pressure would a sample of CO2(g) behave least like an ideal gas? C
1) 0°C and 100 kPa
2) 150°C and 100 kPa
3) 150°C and 300 kPa
4) 0°C and 300 kPa
Answers
The conditions of temperature and pressure in which a sample of CO₂ (g) would behave least like an ideal gas are high temperature and high pressure; 150°C and 300 kPa, option C
What are ideal gases?An ideal gas is any gas whose molecules obey the gas law and whose behavior follows the ideal gas behavior.
The behavior of ideal gases is as follows:
the intermolecular forces of attraction between the gases are negligiblethe collision of the molecules of the gas is perfectly elasticFor a given gas to behave as an ideal gas, it must be under conditions of extremely high temperatures and low pressure.
Learn more about ideal gases at: https://brainly.com/question/27870704
#SPJ1
What is one energy transformation that is taking place in the photo?
radiant energy to thermal energy
thermal energy to nuclear energy
chemical energy to thermal energy
radiant energy to chemical energy

Answers
Answer:
Radiant energy to chemical energy
Explanation:
Got it right on edge 2021
One energy transformation that is taking place in the photosynthesis process is radiant energy to chemical energy.
What is energy transformation?Energy transformation is the process in which one form of energy converted into another form of energy.
What is photosynthesis process?Photosynthesis process is defined as the process in which plants makes their own food by using the carbon dioxide, water and sunlight in the chlorophyll.
The balanced chemical equation of photosynthesis process is
6CO2 + 6H2O → C6H12O6 + 6O2.
Since, it have 6 atoms of carbon, 12 atoms of hydrogen and 18 atoms of oxygen on both side. So, it is balanced.
Since, sunlight which it take is in the form of radiant and convert it into chemical energy.
Thus, we concluded that the energy transformation that is taking place in the photosynthesis process is radiant energy to chemical energy.
learn more about photosynthesis process:
https://brainly.com/question/26568636
#SPJ2