Classify the following substances as strong electrolytes, weak electrolytes, or nonelectrolytes:
(a) Sodium permanganate (NaMnO₄)
Answers
Sodium permanganate (NaMnO₄) is strong electrolytes
A strong electrolyte is an electrolyte that dissolves almost completely in water. An example of a strong electrolyte is Hydrogen Chloride (HCl).
What happens to water when potassium permanganate is added?A thick purple solution emerges in the water at the bottom of the beaker due to the random movement of potassium permanganate particles. The purple solution will gradually diffuse into the remaining water in the beaker, resulting in a less thick but equally tinted purple solution.
If the molar amounts are the identical, both variants are equally effective. But since sodium permanganate is more soluble (40% vs. 5%), it is more adaptable and less difficult to utilise. Potassium permanganate is often less expensive and, because it cannot be produced into a highly concentrated solution, is potentially safer.
learn more about electrolyte refer:
https://brainly.com/question/17089766
#SPJ4
Related Questions
The diagram below shows two substances. What name is given to bond X?

Answers
Answer:
single covalent bond
Explanation:
The bond formed between two atoms each sharing one electron is called single covalent bond
The figure 1 is the structure of diamond. The type of bond in diamond is covalent sp³ bond. DIamond exists in hexagonal arrangement of carbon atoms.
What is allotrope?Allotropes are substances formed from a single elements with different number in various size and shape. Carbon exhibit catenation property which means it can forms chains with other carbon in any large number.
The allotropes of carbon are diamond, graphene and fullerene. Diamond is hexagonal arrangement of carbons atom bonded covalently in sp³ hybridisation.
In graphene as shown in the second figure, bonded carbons atoms are arranged as layers where carbon is in sp²hybridisation. In the case of fullerenes, there are both hexagonal and pentagonal rings adjacent to each and are formed by 60-70 carbons.
In all these allotropes, the bond type between two carbons atoms is covalent. Covalent bond is formed by sharing of electrons between the two atoms.
To find more about diamond, refer the link below:
https://brainly.com/question/1120149
#SPJ2
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
If the gas you collected for the unknown gas lab was Carbon Dioxide, what other procedure can you perform to provide additional evidence that it might be carbon dioxide. O Measure the Density of the gas at room temperature and compare to the density of carbon dioxide at room temperature. Check the pH (acidity) of water used to collect the gas (carbon dioxide bubbled in water produces a slight acid solution) Flammability test (Carbon dioxide is not flammable, test would rule out flammable gases similar MW such as propane) All answers are correct here.
Answers
All answers are correct here. The density test can provide evidence based on the known density of carbon dioxide, the pH test can confirm the presence of an acid solution
And the flammability test can rule out the presence of flammable gases with similar molecular weight.
In addition to the tests mentioned, there are several other procedures that can be performed to confirm the presence of carbon dioxide gas:
Limewater test: Carbon dioxide reacts with limewater to form a cloudy precipitate of calcium carbonate.
Infrared spectroscopy: Carbon dioxide has a characteristic absorption band at 2349 cm-1 in the infrared region.
Mass spectrometry: Mass spectrometry can be used to determine the molecular weight of the gas and compare it to the known molecular weight of carbon dioxide.
Gas chromatography: Gas chromatography can be used to separate and identify the different components of a gas mixture.
Overall, a combination of these tests can be used to provide strong evidence for the presence of carbon dioxide gas in a sample.
To learn more about carbon dioxide here:
https://brainly.com/question/3049557
#SPJ11
Which material has selective permeability a sponge or a shovel?.
Answers
a sponge because it lets in some of the dirt and mess but it doesn't let in all of it as in a shovel doesn't really take in anything
A sponge has selective permeability, while a shovel does not. Selective permeability refers to the ability of a material or membrane to allow certain substances to pass through while restricting the passage of others.
In the case of a sponge, it has a porous structure with small openings that can absorb and hold liquids, while allowing water to pass through. This selective permeability enables a sponge to soak up and retain liquids while filtering out larger particles.
A shovel is typically made of solid materials like metal or plastic, which do not possess selective permeability. A shovel's main purpose is to scoop and transport materials such as soil, sand, or snow, but it does not have the ability to selectively allow substances to pass through its structure.
To learn more about the permeability, follow the link:
https://brainly.com/question/32006333
#SPJ6
Give the symbol balanced equation for the reactions below. Ensure states are used.
a) Carbonic acid forming when a hydrogen ion reacts with a bicarbonate ion in a reversible reaction.
Answers
Answer:
\({ \rm{2H {}^{ + } _{(aq)} + CO {}^{2 - } _{3(aq)} \: \: \: {}^{ { \huge{\dashrightarrow} }} _{ \huge{ \dashleftarrow}} }} \: \: { \rm{H _{2} CO _{3(aq)} }}\)
Which is TRUE about 'acidified' water acting on rocks?
Please give 1 answer.
A.
Peaty soil water does not cause weathering
B.
Limestone is eroded faster than basalt rock by this type of weathering
c.
It is an example of physical weathering
D.
Nearly neutral water will not weather rocks
Answers
In order for something to be scientific, it needs to be ________________, ___________________, _________________, and __________________.
Answers
Answer:
In order for something to be scientific it needs to be:
a) observable
b) replicable;
c) falsifiable,
d) testable
Explanation:
A) Science does not deal with non-obserbale or faith based phenomena. If it's not observable, it probably imaginary, therefore unscientific.
B) a scientists must be able to replicate phenomena under controlled environments in order to study it more closely.
C) a scientific phenomena must allow for the formation of a hypothesis. That is, if it is scientific, then the phenomena should allow for an explanation (no necessarily the right one) to be postulated at an experiment
D) the hypothesis surrounding the scientific pheonomena must be testable.
Cheers!
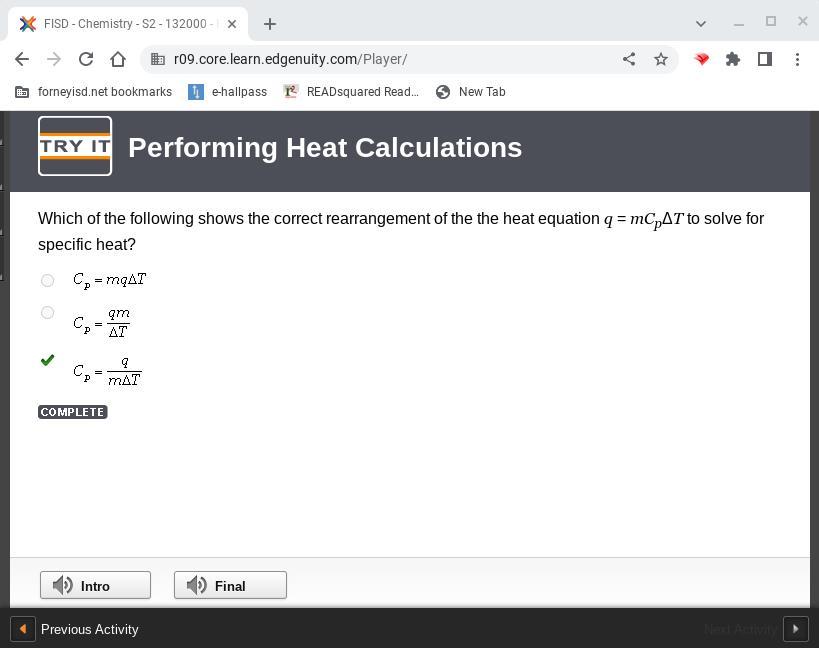
Which of the following shows the correct rearrangement of the the heat equation q = mCpΔT to solve for specific heat?
Answers
Answer:
c
Explanation:
Answer:
The Answer is the last one
Explanation:
choose all constitutional isomers that have molecular formula c4h8o.
Answers
The constitutional isomers that have the molecular formula C₄H₈O are Butanone, Butanal, 2-butanone, Pentan-3-one, Hexanal, and Propanal.
Constitutional isomers are defined as compounds that have the same molecular formula but a different arrangement of atoms within the molecule.
The molecular formula for the given problem is C₄H₈O.
Constitutional isomers for this compound are as follows:
Butanone, Butanal2-butanone, Pentan-3-one, Hexana, lPropanal.
The molecular formula for each compound has four carbon atoms, eight hydrogen atoms, and one oxygen atom, and they have different structures as well.
The first compound, Butanone, has two carbon atoms in the chain with an oxygen atom double bonded to one of them. This compound is a type of ketone and is also known as methyl ethyl ketone.
The second compound is Butanal, which is an aldehyde with two carbon atoms in the chain and a double bond to oxygen.
The third compound, 2-butanone, has a carbonyl group between the second and third carbon atom of the chain, whereas the fourth compound, Pentan-3-one, has a carbonyl group between the third and fourth carbon atoms of the chain.
The fifth compound is hexanal, which is an aldehyde that contains six carbon atoms in the chain. The last compound is propanal, which is an aldehyde with a chain containing three carbon atoms.
The carbon and hydrogen atoms in each compound are arranged differently, giving rise to the phenomenon known as constitutional isomerism.
Therefore, the constitutional isomers that have the molecular formula C₄H₈O are Butanone, Butanal, 2-butanone, Pentan-3-one, Hexanal, and Propanal.
The question should be:
Choose all constitutional isomers that have molecular formula C₄H₈O: Butanone, Butanal, 2-butanone, Pentan-3-one, Hexanal, and Propanal.
Learn more about isomers at: https://brainly.com/question/2705480
#SPJ11
the rate constant for a reaction is found to be 0.15 m-1s-1. if the initial concentration of the reactant is 0.30 m, how long (in seconds) does it take for the concentration to decrease to 0.15 m?
Answers
A reaction's rate constant is discovered to be 0.15 . If the initial concentration of the reactant is 0.30 m, 22.2 sec of time does it take for the concentration to decrease to 0.15 m.
1/[A]t=kt+1/[A]o
t=(1/[A]t−1/[A]o)/k=(1/0.15−1/0.30)/0.15=22.2seconds
Mathematically, time and other physical quantities can be coupled to create additional ideas like motion, kinetic energy, and time-dependent fields. The basis of recordkeeping is timekeeping, a complex of technological and scientific challenges. Time is change, or the span of time during which change takes place with motion. Without a change, it is impossible to detect the passage of time. Comparison with a standard is used to calibrate the length of time or change.The SI unit of time is called the second, abbreviated as s. Time can be used to quantify, compare, or even order events based on their duration or the intervals between them. One method of making efficient use of resources is to use time study and motion study, which enhance output and performance.
Learn more about time here:
https://brainly.com/question/15357495
#SPJ4
Can you use a meat thermometer to take your temperature?
Answers
Answer:
It won't work as well. You can try though, it won't hurt anything
What are the 4 types of characterization?.
Answers
Answer:
There are actually five, and there's an easy way to remember them.
Physical Description
Action
Inner thoughts
Reactions
Speech
P.A.I.R.S
This will basically help you with any story you come up with
what can a food scientists take advantage of with the background of chemistry?
Answers
Food scientists work with the chemistry of ingredients in food to improve the quality and stability of the food. They study the use of chemical flavors, thickening agents, stabilizers and preservatives and apply their knowledge to improve existing food products and develop new ones.
GUYS IF YOU GET THE THING ABOUT THE CHILD PERV I WOULD WATCH OUT BUT SOME OF THEM ARE JUST DOING IT TO GET FREE POINTS SO REPORT THEM!! :)
Pls brainliest! :)
Na2CO3 is a/an ____________ compound where its electrons are _______. *
1.covalent, shared
2.covalent, gained/lost
3.ionic, gained/lost
4.ionic, shared
Answers
Answer:
Answer is 3 because NaCo3 because NaCo3 is ionic compound and electrons of ionic compound are gained / lost
Which element has the following electron configuration notation
Answers
The element which has the following electron configuration notation 1s^22s^22p^63s^23p^1 is referred to as aluminium.
What is Aluminium?This is referred to a element which has an atomic number of 13 and is regarded as a metal which has a white or silver color. This type of metal has a very low density when compared to the other ones which are present in nature.
It is therefore the reasons why it is used to make substances such as foil due to its soft and malleable property.
This is therefore the reason why Aluminium was chosen as the most appropriate choice.
Read more about Aluminium here https://brainly.com/question/246454
#SPJ1
a guitar string vibrates at a frequency of 5 Hz and has a wavelength of 3 m. what is the wave speed?
Answers
V=2*3/5
V=6/5
V=1.2ms-1
what is the reason for using a cacl2 drying tube in the reflux setup? draw a general reaction scheme to support your answer. (2 pts)
Answers
A CaCl₂ drying tube is often used in a reflux setup to remove any water vapor that may be present in the reaction mixture or the reflux condenser.
Water can interfere with the reaction or cause unwanted side reactions, so it is important to keep the reaction mixture dry.
The CaCl₂ drying tube works by absorbing any water vapor that passes through it, leaving the reaction mixture dry. The reaction scheme for this process can be represented as follows:
CaCl₂ (s) + H₂O (g) → CaCl₂•nH₂O (s)
The CaCl₂ drying tube contains solid CaCl₂, which has a high affinity for water. As water vapor passes through the drying tube, it reacts with the CaCl₂ to form a hydrate, CaCl₂•nH₂O. This reaction removes the water vapor from the reaction mixture, ensuring that the reaction proceeds as intended.
The CaCl₂ drying tube is typically placed between the reaction vessel and the reflux condenser, so that any water vapor that is produced during the reaction is removed before it reaches the condenser.
This helps to prevent the condenser from becoming clogged with water, which could interfere with the reflux process. Overall, the CaCl₂ drying tube is an important component of the reflux setup, as it helps to ensure that the reaction proceeds smoothly and efficiently.
To know more about reflux setup here
https://brainly.com/question/28828626
#SPJ4
How many liters of space will a 70.0 grams sample of CO2 occupy?
Answers
35.6 liters of space will a 70.0 grams sample of CO2 occupy.
What do you mean by molar mass ?Molar mass is defined as the mass of a given substance divided by the amount of that substance, expressed in grams per mol.
To calculate a molecule's molecular mass, multiply the subscript by the atomic mass of each element in the molecule, then add the masses of all the elements in the molecule.
The molar mass of carbon dioxide is 44.01 g/mol.
The volume of a mole of ideal gas at STP is 22.4 L,
Therefore, the volume of 70.0 g = ?
= (70.0g) / (44.01 g/mol) (22.4 L/mol)
= 35.6 L
Thus, 35.6 liters of space will a 70.0 grams sample of CO2 occupy.
To learn more about the molar mass, follow the link;
https://brainly.com/question/12127540
#SPJ1
Gasoline is a product of crude oil, compressed organic matter formed from accumulations of algae millions of years ago. Some places are now substituting ethanol in place of gasoline. Ethanol is formed by fermenting certain foods like corn with yeast, then extracting the alcohol that is given off. Which of these energy sources is nonrenewable?
Answers
Answer:
Gasoline/oil is not renewable
how many milliliters of an aqueous solution of 0.235 m silver fluoride is needed to obtain 9.72 grams of silver fluoride ?
Answers
326.0266 milliliters of an aqueous solution of 0.235 m silver fluoride is needed to obtain 9.72 grams of silver fluoride.
Given data,
mass = 9.72 g
molar mass of iron fluoride = 126.866 g/mol
molarity = 0.235 m
Molarity = \(\frac{w_{solute} }{m_{solute} }\)×\(\frac{1000}{V}\)
0.235 = \(\frac{9.72}{126.866 g/mol}\)×\(\frac{1000}{V}\)
0.235 = 0.076616 × 1000 / V
0.235 = 76.6162/v
Volume = 326.0266 ml
A solution in which water serves as the solvent is called an aqueous solution. The most common way to represent it in chemical equations is to add (aq) to the appropriate chemical formula. For instance, the formula for a solution of table salt, or sodium chloride (NaCl), in water is Na+(aq) + Cl (aq). The word aqueous, which derives from the Greek aqua, denotes that anything is connected to, resembles, or is dissolved in water. Water is a common solvent in chemistry since it is a great solvent and abundant in nature. Since water is usually used as the experiment's solvent, unless otherwise stated, the term "solution" refers to an aqueous solution.
Learn more about Aqueous solution here:
https://brainly.com/question/14097392
#SPJ4
What are three elements in the same period
Answers
Explanation:
lithium hydrogen sodium oxygen
David says, "Pure honey has nothing else added." Susan says, "The honey is not really pure. It is a mixture of many different substances." Who is right? Explain your answer.
Answers
Answer:
Susan is right
Explanation:
Honey is a natural substance extracted from bees. Like every other natural substance, honey is a mixture of many different substances. An analysis of the so called 'pure honey' will reveal that many chemical species compose the natural substance called honey.
Hence Susan is right when she says that honey is not really pure but a mixture of substances.
a sample of xenon gas occupies a volume of 6.56 l6.56 l at 499 k.499 k. if the pressure remains constant, at what temperature will this same xenon gas sample have a volume of 3.38 l?
Answers
The temperature at which the xenon gas will have a volume of 3.38 litres is calculated to be 257.1 K.
How to calculate temperature in relation to volume when pressure is constant?Charles' law, which states that, provided the pressure is constant, the volume occupied by a particular quantity of gas is directly proportional to the absolute temperature. According to the law, volume increases with increasing temperature and decreases with decreasing temperature, applies when pressure is constant. The relationship equation for the Charles's law can be written as-
\(V_{i}\)/\(T_{i}\)= \(V_{f}\)/\(T_{f}\)
where,
\(V_{i}\) = initial volume
\(T_{i}\) = initial absolute temperature
\(V_{f}\) = final volume
\(T_{f}\) = final absolute temperature
According to the given question,
\(V_{i}\) = 6.56 litres, \(T_{i}\) = 499 Kelvin, \(V_{f}\) = 3.38 litres, \(T_{f}\) = ?
The temperature is given in Kelvin so we can put it as it is. So, on applying the given values in the formula:
6.56 / 499 = 3.38 / \(T_{f}\)
Now, for final temperature we can rearrange the equation to get-
\(T_{f}\) = (3.38 × 499) / 6.56
\(T_{f}\) = 257.1 K
To know more about Charles's law, visit:
https://brainly.com/question/16927784
#SPJ4
7/which is true regarding excretion when tubular urine is more alkaline? a. both weak acids and weak bases are excreted more rapidly. b. weak acids are excreted more rapidly, and weak bases are excreted more slowly. c. weak acids are excreted more slowly, and weak bases are excreted more rapidly. d. both weak acids and weak bases are excreted more slowly.
Answers
When tubular urine is more alkaline, weak acids are excreted more slowly and weak bases are excreted more rapidly. This is because the pH of the urine affects the ionization state of these compounds, which in turn affects their ability to be excreted.
In an alkaline environment, weak acids will be more ionized and less likely to be excreted. This is because ionized molecules are less likely to be reabsorbed by the tubular cells and more likely to be excreted into the urine. On the other hand, weak bases will be less ionized and more likely to be excreted. This is because non-ionized molecules are more likely to diffuse across the tubular membrane and be excreted.
Therefore, option (c) is true: weak acids are excreted more slowly, and weak bases are excreted more rapidly when tubular urine is more alkaline. It is important to note that this is the opposite of what happens in acidic urine, where weak acids are excreted more rapidly and weak bases are excreted more slowly.
To learn more about weak acids refer to:
brainly.com/question/22104949
#SPJ4
please help, i will give free kfc. i work in kfc.

Answers
Answer:Sexual reproduction can be described as the method of reproduction in which the offsprings produced will have half the chromosomes as compared to the parent cell. The other half of the chromosomes to make a complete set would arise from the other parent. In this way, the offspring produced will carry half of the chromosomes from the female parent and half from the male parent.
Crossing over and independent assortment are two phenomenons of meiosis due to which genetic diversity occurs and the offsprings born are not exactly similar to the parent cell.
Explanation:
A farmer is breeding his best livestock is and example of?
Answers
Answer:
This is an example of selective breeding
Choose the statement that describes what will happen to pH as hydrogen ion concentration increases. Multiple Choice pH will not change. О O pH will increase pH will decrease.
Answers
The statement that describes what will happen to pH as hydrogen ion concentration increases is: "pH will decrease."
The pH of a solution is a measure of its acidity, and it is defined as the negative logarithm of the hydrogen ion concentration (H+). As the hydrogen ion concentration increases, the pH of the solution decreases, indicating an increase in acidity. Conversely, as the hydrogen ion concentration decreases, the pH increases, indicating a decrease in acidity.
The pH scale is a measure of the acidity or basicity (alkalinity) of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are considered acidic, while solutions with a pH greater than 7 are considered basic or alkaline.
The hydrogen ion concentration (H+) of a solution is an important factor in determining its pH. As the concentration of hydrogen ions increases, the solution becomes more acidic, and the pH decreases. Conversely, as the concentration of hydrogen ions decreases, the solution becomes less acidic, and the pH increases.
It's important to understand the pH of solutions because it can have a significant impact on chemical reactions and biological processes. For example, the pH of the human body is carefully regulated, and deviations from the normal pH range can lead to serious health problems. Similarly, the pH of soil, water, and other environments can have a profound effect on the survival and growth of plants, animals, and microorganisms.
Learn more about pH scale here:
https://brainly.com/question/1596421
#SPJ4
How many molecules are in 83.2 g of chlorine gas (Cl2)?
Group of answer choices
1.42 x 10^24 molecules
1.78 x 10^28 molecules
7.04 x 10^23 molecules
14.2 x 10^24 molecules
Answers
Answer:
try all of them
1.42 x 10^24 molecules
1.78 x 10^28 molecules
7.04 x 10^23 molecules
14.2 x 10^24 molecules
Explanation:
PLS HELP ASAP!!!!!! WILL GIVE BRAINLIEST!!!!!!!!!!
Write a summary paragraph for each part discussing this experiment and the results. Use the following questions and topics to help guide the content of your paragraph. Part 1 What was your hypothesis? According to your data, do you think your hypothesis was correct? (Be sure to refer to your data when answering this question.) Summarize any difficulties or problems you had in performing the experiment that might have affected the results. Describe how you might change the procedure to avoid these problems. Be sure to submit your data along with your paragraph. Part 2 What effect did the temperature have on the viscosity of the honey? (Be sure to refer to your data when answering this question.) Give at least two practical examples where knowledge of viscosity is important.
Answers
Answer:
Explanation:
Dang which subject is this history or science
4. C2 JUN 09 Q7c
Propene reacts with hydrogen bromide to give 2-bromopropane as the major product.
(1) Using the reaction scheme below, show the mechanism of the reaction using curly
arrows and full negative and positive charges as appropriate.
(2)
H,C
H
c=c
H
H
H-Br
CH, H
H-C-C-H
Br H
CH, H
1 1
H-C-C-H
Br H
2-bromopropane
(ii) State briefly, why 2-bromopropane, rather than 1-bromopropane, is the main product
of this reaction.
[1]
Answers
Explanation:
(1) The mechanism of the reaction between propene and hydrogen bromide to give 2-bromopropane is as follows:
Protonation of the alkene: A proton from hydrogen bromide (HBr) attacks the alkene, forming a carbocation intermediate.
Bromine addition: Bromine (Br) adds to the carbocation intermediate to form an intermediate bromonium ion.
Deprotonation: A proton from the bromonium ion is removed by a water molecule or another molecule, producing the final product 2-bromopropane.
The mechanism can be represented using curly arrows as follows:
H,C
H
c=c
H
H
H-Br
CH, H
H-C-C-H
Br H
CH, H
1 1
H-C-C-H
Br H
2-bromopropane
(2) 2-bromopropane is the main product of this reaction because of the stereochemistry of the reaction. When the carbocation intermediate forms, the bromine atom has a preference for adding to the face of the alkene that has the least number of hydrogen atoms. This leads to the formation of the 2-bromopropane, which has the bromine atom attached to the carbon atom with two hydrogen atoms. On the other hand, the formation of 1-bromopropane, which has the bromine atom attached to the carbon atom with three hydrogen atoms, is less favorable. This is why 2-bromopropane is the main product of this reaction.