Choose the situation below that would result in an endothermic ΔHsolution.
a.When <
b.When >
c.When is close to
d.When >>
e.There isn't enough information to determine.
Answers
An endothermic ΔHsolution is a solution where heat is absorbed or taken in. This means that the temperature of the system decreases as heat is being absorbed. In terms of the given situations, option a is the most likely scenario that would result in an endothermic ΔHsolution.
This is because when the temperature of the solution is lower than the temperature of the surrounding environment, the solution would absorb heat in order to reach thermal equilibrium. This would result in an endothermic reaction as heat is being absorbed by the solution. Options b and d suggest that the surrounding environment is cooler than the solution, which means that heat would be released or given off, resulting in an exothermic reaction. Option c suggests that the temperature of the solution and the surrounding environment are similar, which means that there would be little to no heat transfer. Therefore, the most likely situation that would result in an endothermic ΔHsolution is when the temperature of the solution is lower than the temperature of the surrounding environment.
To know more about Endothermic visit:
https://brainly.com/question/11902331
#SPJ11
Related Questions
A student heats a 10 g iron nail (specific heat of 464 j/(kg c) ) with a flame. the nail is then put into 100 g of water at 10 c. the water temperature rose to 20 c, what was the initial temperature of the nail?(specific heat of water is 4186 j/(kg c) )
Answers
A student put a 10 g iron nail into 100 g of water. The initial temperature of the nail was 922 ⁰C
According to the law of conservation of heat energy:
released heat = received heat
The amount of released or received heat energy is given by:
Q = c m Δt
Where:
c = specific heat
m = mass
Δt = temperature difference
In the given problem, the nail releases heat while the water receives heat.
Released heat: (nail)
Q1 = c m Δt
= 464 x 0.010 x (tn - 20)
= 4.64 tn - 92.8
Where tn is the initial temperature of the nail.
Received heat: (water)
Q2 = c m Δt
= 4186 x 0.1 x (20 - 10)
= 4186
released heat = received heat
4.64 tn - 92.8 = 4186
4.64 tn = 4,278.8
tn = 922
Hence, the initial temperature of the nail is 922 ⁰C
Learn more about heat transfer here:
https://brainly.com/question/25603269
#SPJ4
Given the functions f(x) = x2 + 6x - 1 and h(x) = 2x2 - 4x + 3. What is the vertex for each?
Answers
Atoms was determined to be 143 picometers. What is the percent error of this measured value?
Answers
Answer:
9.1% error
Explanation:
Full Question:
A measured value for the atomic radius of platinum atoms was determined to be 143 picometers. What is the percent error of this measured value?show with arrows how size changes in both a period and a group. b) also show with arrows how electron affinity changes in both a period and a group.
Answers
a) Size decreases in a period from left to right (→) and increases in a group as you move down (↓). (b) Electron affinity changes increasingly in both a period and a group from left to right (→), and upwards (↑), respectively.
(a) Size changes in a period and a group can be represented with arrows as shown below:
Period:
→ → → → → → →
(Li) (Be) (B) (C) (N) (O) (F)
As we move from left to right in a period, the size of the atoms decreases. This is because the number of protons in the nucleus increases, which leads to a stronger attraction between the nucleus and the electrons, causing the size to decrease.
Group:
↓
(Li)
↓
(Na)
↓
(K)
↓
(Rb)
As we move down a group, the size of the atoms increases. This is because the number of energy levels increases, which leads to a larger distance between the nucleus and the outermost electrons, causing the size to increase.
b) Electron affinity changes in a period and a group can be represented with arrows as shown below:
Period:
→ → → → → → →
(Li) (Be) (B) (C) (N) (O) (F)
As we move from left to right in a period, the electron affinity increases. This is because the atoms have a stronger attraction for electrons as the number of protons in the nucleus increases.
Group:
↓
(Li)
↓
(Na)
↓
(K)
↓
(Rb)
As we move down a group, the electron affinity decreases. This is because the atoms have a weaker attraction for electrons as the number of energy levels increases and the distance between the nucleus and the outermost electrons increases.
Learn more about Electron affinity here: https://brainly.com/question/1287606.
#SPJ11
If you run 3.2 miles, how many kilometers did you run?
Answers
how it helps ^^
Which property of water allows water to stick to the soap
A.polarity
B.cohesion
C.adhesion
D. Surface tension and buoyancy
E. Capillary action
Answers
Answer:
Due to polarity of the soap
Gold and Platinum are found in free state in nature-Give reason.
Answers
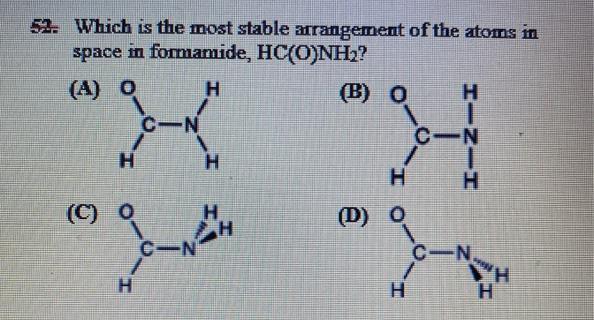
which is the most stable arrangement of the atoms in space in formamide, hc(o)nh2?
Answers
The most stable arrangement of the atoms in space in formamide, HC(O)NH₂, is the trans configuration. Thus, Option A holds true.
Formamide, HC(O)NH₂, is an amide molecule that can exist in two different configurations: the cis configuration and the trans configuration. The cis configuration is where the hydrogen atom attached to the carbon atom and the hydrogen atom attached to the nitrogen atom are on the same side of the molecule.
The trans configuration is where these two hydrogen atoms are on opposite sides of the molecule. The trans configuration is the most stable arrangement of the atoms in space in formamide because it minimizes the steric repulsion between the two hydrogen atoms.
Steric repulsion occurs when atoms or groups of atoms are in close proximity to each other and their electron clouds overlap, causing them to repel each other. By having the two hydrogen atoms on opposite sides of the molecule, the steric repulsion is minimized, making the trans configuration the most stable arrangement of the atoms in space in formamide.
Therefore, A is correct.
Learn more about Formamide brainly.com/question/30687590
#SPJ11
nitrogen monoxide is produced by combustion in an automobile engine. what volume of nitrogen dioxide is produced when 12.0 liters of nitrogen monoxide react according to the following reaction? (all gases are at the same temperature and pressure.)
Answers
41.2 litres of Nitrogen dioxide will be produced.
28.9g NO x (1 mol NO / 30.01g NO) = 0.963 mol NO
0.963 mol NO x (1 mol O2 / 1 mol NO) = 0.963 mol O2
0.963 mol O2 x (31.98g O2 / 1 mol O2) = 30.8g O2
30.8 grams of oxygen gas are required for the complete reaction of the 28.9 grams of nitrogen monoxide.
Charles' law states that the volume of a given gas sample under constant pressure is precisely proportional to its absolute temperature. Boyle's law states that, for a given amount of gas and constant temperature, the volume is inversely proportional to the pressure.
To know more about volume of gas, please refer:
https://brainly.com/question/25736513
#SPJ4
Select the correct answer. Which statement is true of a chemical change? A. It involves changes in the molecular structure. B. It involves changes in phase but not changes in the molecular structure. C. It involves dissolving one substance into another. D. It involves a change in the state, or phase, of a substance. E. It involves the process of separating two or more dissolved substances.
Answers
What aspects of thermodynamics can an enzyme not change?
Answers
The aspects of thermodynamics the enzyme can not change are alter the overall energy balance of a chemical reaction and overall direction of a reaction
Firstly, enzymes cannot alter the overall energy balance of a chemical reaction, the first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. Enzymes can speed up reactions by lowering the activation energy, but they cannot change the total energy input or output.
Secondly, enzymes cannot change the overall direction of a reaction, as dictated by the second law of thermodynamics, this law states that natural processes tend to increase the entropy (disorder) of the system. If a reaction is not thermodynamically favorable (i.e., it would result in a decrease in entropy), enzymes cannot make it occur spontaneously. They can only increase the reaction rate if the reaction is already favorable. In summary, enzymes can speed up the reaction rate and lower activation energy, but they cannot change the total energy balance or the overall direction of a reaction, as these are determined by the laws of thermodynamics.
Learn more about enzymes here:
https://brainly.com/question/31561117
#SPJ11
the microwaves in an oven are of a specific frequency that will heat the water molecules contained in food. (this is why most plastics and glass do not become hot in a microwave oven - they do not contain water molecules). this frequency is about 3 x 109 hz. what is the energy of one photon in these microwaves? for final answer, use scientific notation and round the answer to 2 significant figures.
Answers
The energy of the photon released by the microwave is 19.86 x 10⁻²⁵ J.
The microwave releases photon of a particular energy.
The energy of the photon is given by,
E = hv
Where,
E is the energy of the photon,
h is the Planck's constant,
v is the frequency of the photon.
The value of the Planck's constant is 6.62 x 10⁻³⁴ m²kg/s
The value of the frequency of the photons released by the microwave is 3 x 10⁹ Hz.
Now, putting the values,
E = 6.62 x 10⁻³⁴ x 3 x 10⁹
E = 19.86 x 10⁻²⁵ J.
The energy of the photon is 19.86 x 10⁻²⁵ J.
To know more about Energy of photon, visit,
https://brainly.com/question/19385998
#SPJ4
What is used in facilitated diffusion to assist the transport of sugar and sodium molecules?.
Answers
The facilitated diffusion of carbohydrates, amino acids, and nucleosides through the plasma membranes of most cells is caused by carrier proteins.
What is facilitated diffusion?
The process of spontaneous passive movement of molecules or ions across a biological membrane by particular transmembrane integral proteins is known as facilitated diffusion.
Facilitated diffusion is the process by which molecules move across the plasma membrane with the aid of membrane proteins like channels and carriers. These molecules move down a concentration gradient, which gives them the potential to diffuse into (or out of) the cell.
Hence the answer is carrier protein.
To learn more about carrier protein follow the link:
https://brainly.com/question/29588351
#SPJ4
for each solute, identify the better solvent: water or carbon tetrachloride. you are currently in a sorting module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop. water, h2o carbon tetrachloride, ccl4
Answers
Student question: For each solute, identify the better solvent: water or carbon tetrachloride.
To determine the better solvent for a given solute, consider the general rule "like dissolves like." This means that polar solutes dissolve well in polar solvents, and nonpolar solutes dissolve well in nonpolar solvents.
Water (H2O) is a polar solvent, while carbon tetrachloride (CCl4) is a nonpolar solvent.
1. If the solute is polar, water (H2O) would be the better solvent.
2. If the solute is nonpolar, carbon tetrachloride (CCl4) would be the better solvent.
Remember to identify the solute's polarity first, and then use the "like dissolves like" principle to choose the appropriate solvent.
what is a formula for sodium nitrate
Answers
Answer:
NaNO3
Explanation:
sodium= Na
Nitrogen= N
and there's 3 oxygens = O3
Genetic material is carried the ______________ of a cell
1.vacuoles
2.cytoplasm
3.nucleus
4.cell membrane
Answers
Answer:
3. nucleus
Explanation:
The nucleus contains all the genetic information.
a chemist has acid solutions with concentrations 8% and 14%. he wants to mix some of each solution to get 87 milliliters of solution with a 12% concentration. how many milliliters of each solution does he need to mix together?
Answers
The chemist needs to mix 29 milliliters of the 8% acid solution with 58 milliliters of the 14% acid solution to obtain 87 milliliters of a 12% acid solution.
Let's assume the chemist needs to mix x milliliters of the 8% acid solution and y milliliters of the 14% acid solution to obtain a total volume of 87 milliliters with a 12% concentration.
To solve this problem, we can set up a system of equations based on the amount of acid in each solution.
Equation 1: The total volume equation
x + y = 87 ---(1)
Equation 2: The concentration equation
0.08x + 0.14y = 0.12 × 87 ---(2)
Let's solve this system of equations to find the values of x and y.
From equation (1), we can rewrite it as x = 87 - y and substitute it into equation (2):
0.08(87 - y) + 0.14y = 10.44
Simplifying the equation:
6.96 - 0.08y + 0.14y = 10.44
0.06y = 3.48
y = 3.48 / 0.06
y = 58
Now, substitute the value of y back into equation (1) to find x:
x + 58 = 87
x = 87 - 58
x = 29
Therefore, the chemist needs to mix 29 milliliters of the 8% acid solution with 58 milliliters of the 14% acid solution to obtain 87 milliliters of a 12% acid solution.
To know more about concentration:
https://brainly.com/question/27926477
#SPJ4
Generally, a solution of an organic compound in water will be electrically?
a. nonconductive, b. highly conductive, c. charged, d. highly ionized, e. insulated
Answers
A solution of an organic compound in water will generally be nonconductive. Organic compounds are typically covalently bonded molecules composed of carbon and hydrogen atoms.
Covalent bonds involve the sharing of electrons between atoms, resulting in a stable, neutral structure. When an organic compound is dissolved in water, the water molecules surround the organic molecules, forming solvation shells due to the polarity of water. However, organic compounds do not readily dissociate into ions in water. Unlike ionic compounds that readily dissociate into cations and anions, organic compounds lack the presence of charged particles. Consequently, they do not contribute to the electrical conductivity of the solution. Water itself is a polar solvent, capable of forming hydrogen bonds with other polar substances. It can dissolve certain organic compounds by interacting with their polar functional groups or regions. Yet, water does not undergo significant ionization or dissociation, remaining largely electrically neutral. Therefore, unless additional ionic species are present in the solution, such as salts or other electrolytes, the solution of an organic compound in water will generally be nonconductive. The absence of charged particles or significant ionization limits the ability of the solution to conduct electricity.
Learn more about organic compound here : brainly.com/question/13508986
#SPJ11
What volume of oxygen gas at 320 K and 680 torr will react with 2.50 L of NO gas at the same temperature and pressure
Answers
1.85 liters of oxygen gas will react with 2.50 liters of NO gas at 320 K and 680 torr.
Given:
T = 320 K
Convert the pressure of 680 torrs to atm by dividing by 760 (since 1 atm = 760 torrs):
P = 680 torr / 760 torr/atm
= 0.8947 atm
For NO gas:
P(NO) = 0.8947 atm
V(NO) = 2.50 L
T = 320 K
R = 0.0821 L·atm/(mol·K)
Use the ideal gas law equation:
PV = nRT
Where:
P = pressure (in atm)
V = volume (in liters)
n = number of moles
R = ideal gas constant (0.0821 L·atm/(mol·K))
T = temperature (in Kelvin)
Rearrange the ideal gas law equation to solve for the number of moles:
n = PV / RT
n(NO) = (0.8947 atm × 2.50 L) / (0.0821 L·atm/(mol·K) × 320 K)
n(NO) = 0.1074 mol
According to the balanced chemical equation, the stoichiometric ratio between NO and O₂ is 2:1. Therefore, the number of moles of oxygen required will be half of the moles of NO gas:
n(O₂) = 0.1074 mol / 2
n(O₂) = 0.0537 mol
P(O₂) = 0.8947 atm
T = 320 K
R = 0.0821 L·atm/(mol·K)
n(O₂) = 0.0537 mol
V(O₂) = (n(O₂) R T) / P(O₂)
V(O₂) = (0.0537 mol × 0.0821 L·atm/(mol·K) × 320 K) / 0.8947 atm
V(O₂) = 1.85 L
To learn more about the oxygen, follow the link;
https://brainly.com/question/13905823
#SPJ4
Ormaldehyde, ch₂o, is an important precursor to many chemical products, including melamine resin, a thermosetting plastic material. is the molecule polar?
Answers
Yes, the molecule formaldehyde (CH₂O) is polar. In order to determine the polarity of a molecule, we look at the electronegativity difference between the atoms involved. In the case of formaldehyde, there is a significant difference in electronegativity between carbon (C) and oxygen (O) atoms.
Oxygen is more electronegative than carbon, which means it attracts the shared electrons more strongly. As a result, the oxygen atom in formaldehyde gains a partial negative charge (δ-) while the carbon atom gains a partial positive charge (δ+). Additionally, the geometry of formaldehyde, which has a bent structure, also contributes to its polarity. The presence of a polar bond and the asymmetric distribution of charge in the molecule make formaldehyde polar overall. This polarity has implications for its chemical properties and reactivity.
To know more about thermosetting plastic visit:
https://brainly.com/question/32232170
#SPJ11
pls help me guys please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please

Answers
5) B
6) C
7) B newland, law of octaves
8) A)
Lab Report
Condensation
It’s time to complete your Lab Report. Save the lab to your computer with the correct unit number, lab name, and your name at the end of the file name (e.g., U1_ Lab_Condensation_Alice_Jones.doc).
Introduction
1. What was the purpose of the experiment?
Type your answer here:
2. What were the independent, dependent, and control variables in your investigation?
Type your answer here:
Experimental Methods
1. What tools did you use to collect your data?
Type your answer here:
2. Write your procedure. List each step so that another student could follow the procedure and repeat your experiment.
Type your answer here:
Data and Observations
1. Record your observations in a data table.
Type your answer here:
Conclusions
1. What conclusions can you draw about how the temperature of air affects the time for water vapor to condense when it mixes with warm, humid air? Write an evidence-based claim.
Type your answer here:
2. A cold front is the zone where a cold air mass is replacing a warmer air mass. What do you predict will happen to the weather at a cold front when the air is humid? Use cause-and-effect relationships and evidence from your experiment to support your prediction.
Type your answer here:
Answers
The purpose of the experiment was to determine how the temperature of air affects the time for water vapor to condense when it mixes with warm, humid air.
The independent variable was temperature, the dependent variable was the condensation time, and the control variables were water vapor and air.
The tools used to collect data were a stopwatch and thermometer.
The procedure involved measuring the condensation time for the same amount of water vapor at different temperatures
The data showed that as the temperature of air increases, the amount of water vapor it can hold also increases.
1. As the temperature difference increases, the time for water vapor to condense will decrease.
Evidence-based claim: The rate at which water vapor condenses into liquid droplets when warm, humid air mixes with cool air is directly proportional to the temperature difference between the two air masses.
2. At a cold front, if the air is humid, the relative humidity will increase as the temperature decreases, and the increased relative humidity will cause the water vapor in the air to condense into liquid droplets.
Evidence-based claim: It has been observed in experiments where air masses of different temperatures and relative humidities are introduced into a controlled environment, leading to an increase in cloud formation and precipitation in areas affected by a cold front.
What is the effect of an increase in temperature on the condensation time of water vapor?When warm, humid air mixes with cool air, the water vapor in the warm air may condense into liquid droplets if the cool air cannot hold as much water vapor as the warm air. As the temperature of air increases, the amount of water vapor it can hold also increases.
Therefore, the time for water vapor to condense will be affected by the temperature difference between the warm, humid air and the cool air.
At a cold front, the temperature of the air is decreasing as the cold air mass replaces the warm air mass.
Learn more about condensation at: https://brainly.com/question/678243
#SPJ1
true or false: the molar enthalpy of sublimation of a given substance can be determined if its enthalpies of fusion and vaporization are known.
Answers
The given statement "The molar enthalpy of sublimation of a given substance can be determined if its enthalpies of fusion and vaporization are known" is true.
The molar enthalpy of sublimation of a substance can be determined if its enthalpies of fusion (melting) and vaporization (boiling) are known. The enthalpy of sublimation refers to the energy required to change a substance from the solid phase directly to the gaseous phase, bypassing the liquid phase.
The enthalpy change during sublimation can be calculated by considering the enthalpies of fusion and vaporization. When a substance undergoes sublimation, it first requires energy to melt from the solid phase to the liquid phase (enthalpy of fusion) and then additional energy to vaporize from the liquid phase to the gaseous phase (enthalpy of vaporization). The sum of these two enthalpies represents the overall energy change during sublimation.
Therefore, by adding the enthalpy of fusion and the enthalpy of vaporization, one can determine the molar enthalpy of sublimation for a given substance.
Learn more about molar enthalpy from the link given below.
https://brainly.com/question/32136429
#SPJ4
If 6. 5g of sulfur reacted with oxygen to produce sulfur trioxide. What is the concentration to sulfur trioxide
2S +302→ 2SO3
Answers
According to the question the concentration of sulfur trioxide 0.0054mol/L.
What is trioxide?Trioxide is a chemical compound made up of three atoms of oxygen. It can exist as a solid, liquid or gas depending on the temperature and pressure conditions. It has a formula of O₃ and is also known as ozone. Trioxide is a powerful oxidizer and is corrosive to many materials, particularly organic compounds.
The concentration of sulfur trioxide in this reaction can be calculated using the mass balance equation. The equation is as follows:
Mass of Sulfur Trioxide (g) = (Mass of Sulfur (g) x 2) / Molecular Mass of Sulfur Trioxide
In this case, the mass of sulfur trioxide = (6.5g x 2) / 80.06g = 0.163g
Therefore, the concentration of sulfur trioxide = 0.163g/30.2g = 0.0054mol/L
To learn more about trioxide
https://brainly.com/question/29576083
#SPJ4
Complete Question:
If 6. 5g of sulfur reacted with oxygen to produce sulfur trioxide. What is the concentration to sulfur trioxide 2SO_2 +30_2→ 2SO_3?
What is the pH of a 0.500 M solution of HF (Ka = 6.8 x 10¯4)?
Answers
So,
First we need to write the dissociation reaction and check the initial, change, and final concentrations:
Using the fact that the equilibrium constant can be found with the equation:
We know the value for Ka, so, let's replace it and then solve for x:
The value of x represents the H+ ions concentration. Using the definition of pH:
Therefore, the pH of the solution is 1.74.




Please helpp
a man is found with a severe case of rigor mortis in
the head and neck region. all external conditions
are fair. if the body was found at 12 noon, which
could be a possible tod?
e. 10:30 am
f. 12:30 pm
g. 11:45 pm
h. 6:15 am
Answers
Rigor mortis is the stiffening of the muscles that occurs after death. It usually starts in the head and neck region and then progresses to the rest of the body. The possible TOD (Time of Death) could be 10:30 am.
Correct option is, . 10:30 am.
The severity of rigor mortis depends on various factors such as the age of the deceased, the temperature, and the cause of death. Since the body was found with a severe case of rigor mortis in the head and neck region, it is safe to assume that the person has been dead for a while.
Rigor mortis, the stiffening of muscles after death, typically begins within 2-6 hours of death and progresses from the head down to the feet. In this case, the man is found with rigor mortis in the head and neck region, which indicates that enough time has passed for rigor mortis to begin. Since the body was found at 12 noon, and considering the minimum 2 hours needed for rigor mortis to set in, the most likely time of death is around 6:15 AM, giving approximately 5 hours and 45 minutes for rigor mortis to begin in the head and neck region.
To know more about Rigor mortis visit:
https://brainly.com/question/30765407
#SPJ11
In witch step did a chemical change most likely occur
Answers
Answer:
step 2
hope this helps!
please mark as brainiest<3
(PLZ ANSWER SOON)
What is the total number of joules of heat that must be absorbed to change the temperature of 100 grams of H20 from 25.0°C to 30.0°C
Answers
Answer:
10.5 k.J.
Explanation:
What percent of AgNO3 is silver?
Answers
Answer:
63.499%
Explanation:
Given: K for acetic acid is 1.8 X 10–5You are titrating 0.108 M NaOH into 142.0 ml of acetic acid of unknown concentration. You have an indicator that will change color when equivalence is reached. At equivalence, you have added 72.0 ml of the base. Calculate molarity of the acid. What is the pH of the solution at the equivalence point? Now that you know the molarity of the acid, find pH when you mix 50.0ml of the acid with 75.0 ml of the same NaOH solution. Now you are working with different acid and base, both weak. K for the acid is 2.25 X 10-5. You mix 63 ml of 0.275 M acid with 55.0 ml of a weak base of concentration 0.188 M. Find pH
Answers
Answer:
Explanation:0.493 M NaOH means 0.493 mol NaOH/L
mols
mols = ------ x L
L
mols = M x V
In a titration procedure, 40.57 mL of 0.493 M NaOH solution was used. How many mols NaOH did this volume of NaOH solution contain?
mols = M x V
0.493 mols NaOH
mols = ----------------------- x 0.04057 L
L
mols = 0.0200 mols NaOH