Answers
The Most Appropriate Reagent(S) For The Conversion Of 2-Methylcyclohexanol To 2- Methylcyclohexanone is (A) PCC, CH₂Cl₂.
It is possible that carbonyl compounds will be produced as a byproduct of the oxidation of alcohols using pyridinium chlorochromate in CH₂Cl₂, however, this will depend on the specific type of alcohol. Primary alcohols will react with PCC to produce aldehydes while secondary alcohols will react with PCC to produce ketones, and tertiary alcohols will not react with PCC. So the most appropriate reagents for the conversion of 2-methylcyclohexanol to 2- methylcyclohexanone are (A) PCC, CH₂Cl₂. The reaction scheme along with the structures of reagents and products are attached.
The complete question is attached.
You can also learn about reagents from the following question:
https://brainly.com/question/10378608
#SPJ4

Related Questions
Suppose you were told that the above reaction was a substitution reaction but you were not told the mechanism. Evaluate the following categories to determine the reaction mechanism and then draw the structure of the major organic product.Type of alkyl halide: _________ (methyl, primary, primary benzyl, primary allyl, secondary, secondary benzyl, secondary allyl, tertiary, tertiary benzyl, tertiary allyl.)Type of nucleophile: _________ (good, moderate, poor.)Solvent: _________ (protic, aprotic.)Is the product racemic? _____ (yes, no.)
Answers
Answer:
The alkyl halide is secondary
The nucleophile is a poor nucleophile
The solvent is a protic solvent
The product is racemic
Explanation:
The reaction is shown in the image attached.
Alkyl halides undergo nucleophilic substitution by two mechanisms; SN1 and SN2. The particular mechanism that applies depends on;
I) structure of the alkyl halide
ii) nature of the nucleophile
iii) nature of the solvent
Looking at the reaction under review, we can see from the structure that the alkyl halide is a secondary alkyl halide. A secondary alkyl halide may undergo substitution via SN1 or SN2 mechanism depending on the conditions of the reaction.
If the nucleophile is poor, and the solvent is protic, SN1 mechanism is favoured over SN2 mechanism. Since CH3CH2OH is a poor nucleophile and ethanol is a protic solvent, we expect the reaction to proceed via SN1 mechanism leading to the formation of a racemic product.
The organic product is also shown in the second image attached.
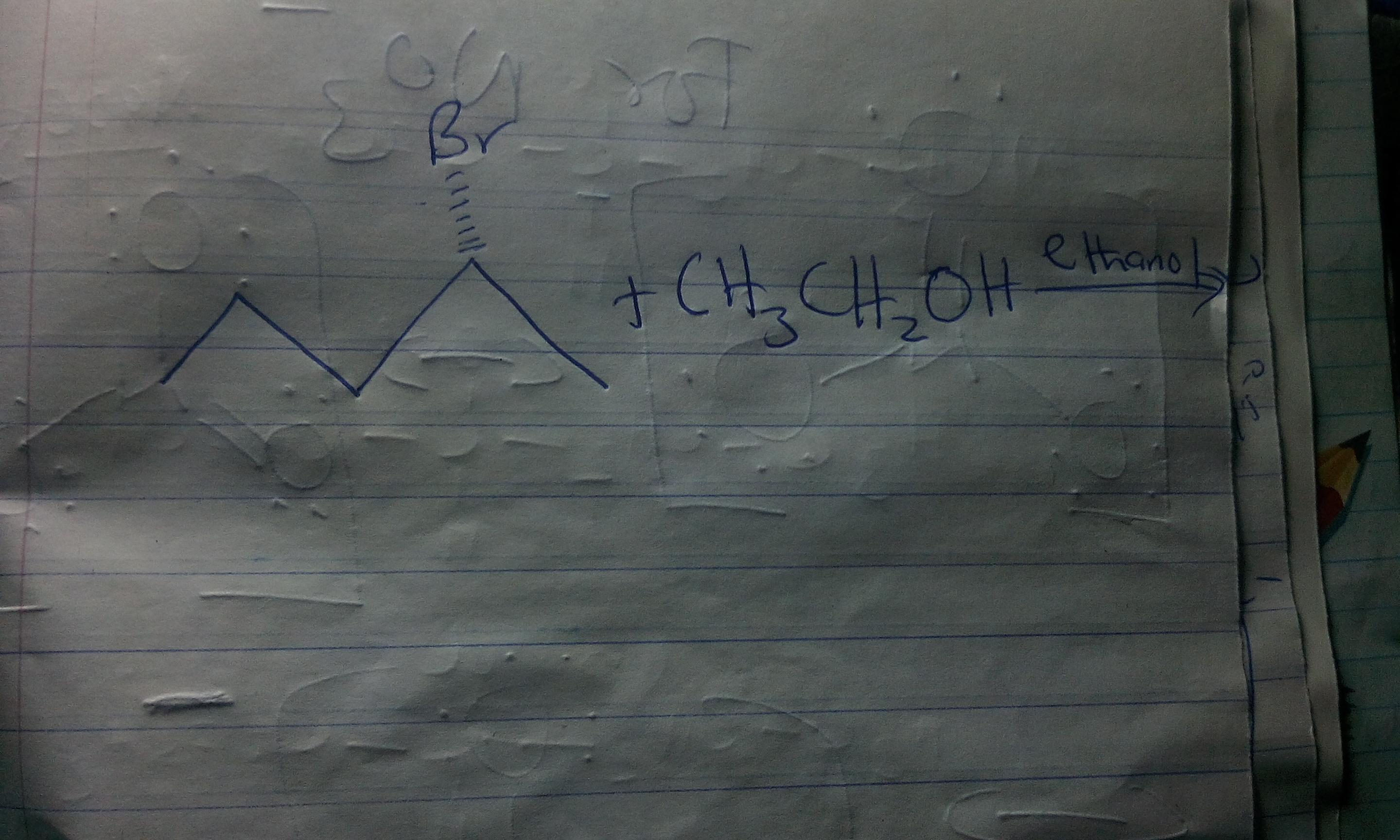
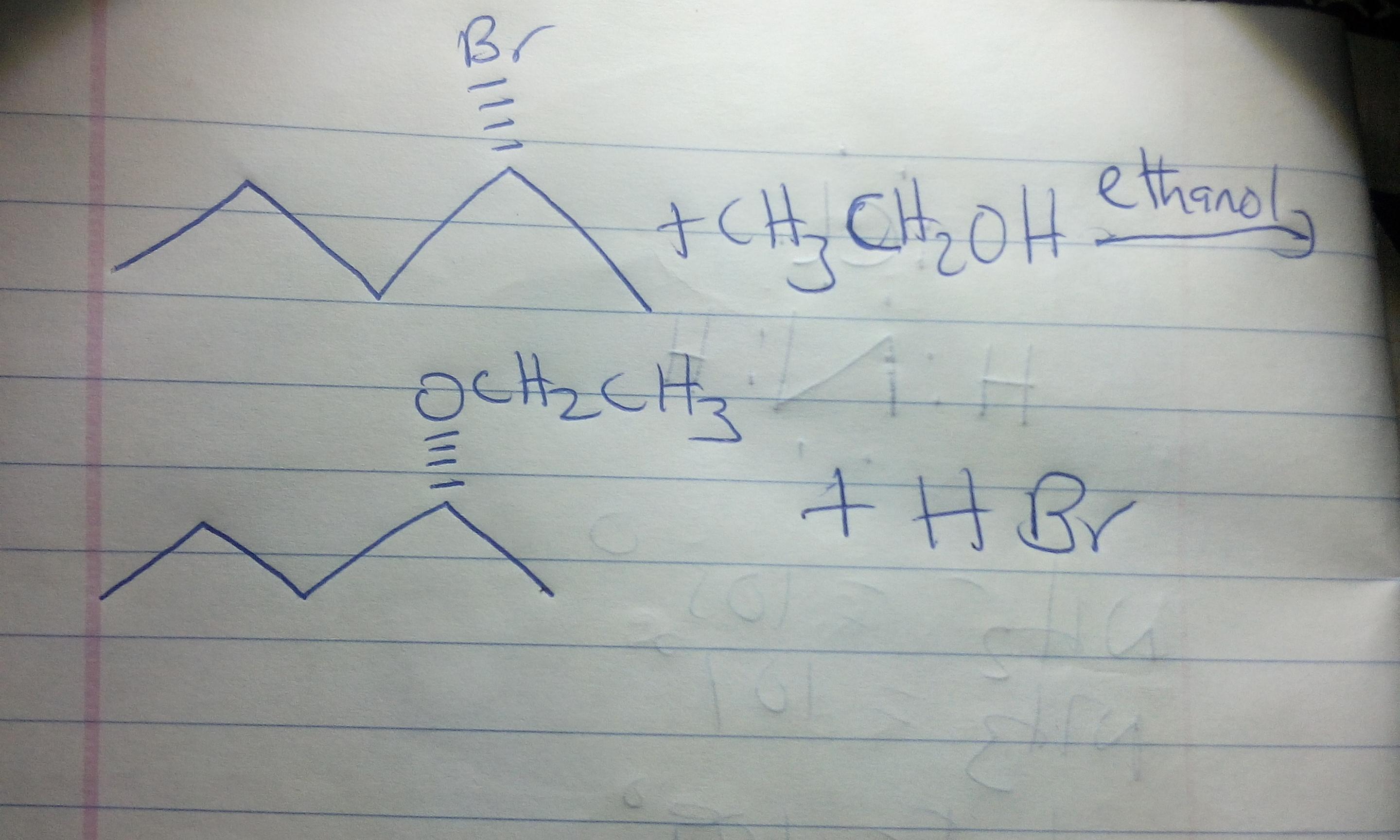
Calculate the mass of 20.0 moles of He (in g)
5.00
1.20 X 1025
1.00
80.0
brainliest to correct answer
Answers
Explanation:
20.0 moles= 80.1 or 80.05g
5.00 moles= 20.0g
1.20×1025moles= 4923.2g
1.00 moles= 4.00g
80.0 moles= 320.2g
How many moles of NaHCO3
are in 27.5 g NaHCO3
?
Answers
(b) Calculate the pH of the following buffer solution.
A solution of 10.0g each of formic acid (CH COOH (Mwt 46g/mol) and Potassium formate (CKHCOO
(Mwt 84g/mol) dissolved in 1.0 litre of water.
(formic acid ka = 1.8 x 104)
Answers
The pH of the buffer solution can be determined using Henderson equation. The pH is obtained as 4.51.
What is buffer solution?A buffer solution is a solution with constant pH and its maintains the pH of a solution upto a desired point. The pH of a buffer solution is determined using Henderson equation as written below:
pH = Pka + ln [A-]/[HA]
Where, [A-] be the concentration of the salt and [HA ] be that of the acid.
Given that 10 g of acid and its salt are dissolved in 1 L solution. Thus,
no.of moles of acid = 10 g / 46 = 0.217 in L means 0.217 M
concentration of salt = 10 g/ 84 g in one L = 0.119 M.
ka = 1.8 × 10⁴
pka = log Ka = 4.25
pH = 4.25 + ln (0.119 / 0.217)
= 4.51
Therefore, the pH of the solution is 4.51.
To find more on buffer solution, refer here:
https://brainly.com/question/24262133
#SPJ1
An apartment with ten floors has a water supply design as shown in the figure.
Answers
Can u give the figure
A Period 2 element has the following successive ionization energies. Identify the element...
1st: 1087 kJ/mol
2nd: 2353 kJ/mol
3rd: 4621 kJ/mol
4th: 6223 kJ/mol
5th: 37832 kJ/mol
6th: 47279 kJ/mol
7th: 55261 kJ/mol
8th: 69875 kJ/mol
Answers
Answer:
9th:08473 kJ/mol
Explanation:
HAHAHAHAHAHAHAHAHAHA
10 cm3 of metallic cylinder has a mass of 39.35 g. The density of this cylinder is
Group of answer choices
3.935 g/cm3
39.35 g/cm3
10 g/cm3
5.935 g/cm3
Answers
Answer:3.935g/cm3
Explanation:39.35 g. /10cm3 = 3.935g/cm3
10 cm³ of metallic cylinder has a mass of 39.35 g. The density of this cylinder is 3.935 g/cm³.
Density is a measure of the amount of mass per unit volume of a substance. It is an intensive physical property, meaning that its value does not change depending on the size of the object.
Density is calculated by dividing an object’s mass by its volume.
The density of water is 1,000 kg/m³ at 4°C. The unit of density is kg/m³ (kilogram per cubic meter). The SI unit of density is kg/m³
Density = mass/volume
= 39.35 g/10 cm³
=3.935 g/cm³.
To know more about density here
https://brainly.com/question/29775886
#SPJ2
Without SALT or SUGAR, does the water conduct electricity?
Answers
What is true regarding compounds?
they are made from two or more atoms of the same element
they are made from the chemical combination of one particular element
they are substances which result from two or more distinct kinds of atoms
Answers
Answer:
Explanation:
A compound is a substance made up of two or more distinct elements that are chemically linked to one another in chemistry. These components mix in predetermined ratios to produce a special set of attributes that are distinct from those of the individual components. Chemical reactions such as combination, breakdown, or exchange reactions can all result in the formation of compounds. Water (H2O), sodium chloride (NaCl), and carbon dioxide (CO2) are a few examples of compounds. Chemistry is based on the understanding of the composition and behavior of compounds, which is essential for many applications in science and technology.
All three statements are true:
A compound can be formed by two or more atoms of the same element. (ex - NaCl)A compound can be formed by a chemical combination of one particular element. (ex - FeS)A compound can be formed by a reaction between two or more distinct kinds of atoms. (ex - \(P_{4}\))Question 9(Multiple Choice Worth 5 points)
(03.07 LC)
How does Word Online indicate a grammar error?
OA line appears underneath.
The word is highlighted.
The word is circled.
The grammar check generates a comment.
Answers
Answer:
A
Explanation:
Base your answer on the equation and diagram below represent an electrochemical cell at 298 K and 1 atmosphere.
When the switch is closed, electrons flow from
A) Ag+(aq) to Mg2+(aq)
B) Mg(s) to Ag(s)
C) Ag(s) to Mg(s)
D) Mg2+(aq) to Ag+(aq)

Answers
When the switch is closed, electrons flow from the solid magnesium electrode, Mg(s) to solid silver electrode, Ag(s).
Electronegativity of metals
Electronegativity of metals refers to the ability of the atoms of metallic elements to attract electrons from the other metallic elements.
Electronegativity increases down the activity series.
Silver (Ag) will have more tendency to attract electron more than magnesium (Mg).
Thus, when the switch is closed, electrons flow from the solid magnesium electrode, Mg(s) to solid silver electrode, Ag(s).
Learn more about electronegativity here: https://brainly.com/question/24977425
#SPJ2
What is the average atomic mass of 10 hydrogen -1 molecules?
Answers
Answer:
1.674 x 10^-23 grams
Explanation:
Hydrogen-1 is called Protium
wikipedia
atomic mass of Protium is 1.00794 amu
sciencedirectcom
atomic mass of 10 Protiums is 10.0794 amu
10.0794 amu in grams is
1.6737236x10^-23 grams
What is the hardest things in the world
Answers
Answer:
Diamond is the hardest things in the world
What type of compound is represented by the graph at right? A. strong base B. strong acid C. weak base D. weak acid

Answers
The type of compound represented by the graph at right is a strong acid (option B).
What is a strong acid?An acid is generally any compound capable of dissociating into its respective constituent ions when in an aqueous solution.
An acid is categorised as strong or weak depending on whether it can dissociate completely or partially. A strong acid dissociates completely in water.
According to this question, HA, when added to water, dissociates into H+ and A- ions, hence, is a strong acid.
Learn more about strong acid at: https://brainly.com/question/29769012
#SPJ1
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
What is the hydronium ion concentration in an aqueous hydrobromic acid solution with a pH of 4.530?
[H3O+] = _____M?
Answers
Answer:
2.951 × 10⁻⁵ M
Explanation:
Let's consider the acid reaction of hydrobromic acid according to Brönsted-Lowry acid-base theory.
HBr(aq) + H₂O(l) ⇒ Br⁻(aq) + H₃O⁺(aq)
Given the pH = 4.530, we can calculate the concentration of the hydronium ion using the following expression.
pH = -log [H₃O⁺]
[H₃O⁺] = antilog -pH = antilog -4.530 = 2.951 × 10⁻⁵ M
Why does an orange look orange?
Ο Α.
It reflects orange light and absorbs other colors.
O B. It absorbs orange light and reflects other colors.
C.
It reflects red and yellow light, only.
O D.
D. It absorbs red and yellow light, only.
Answers
What do scientist use to classify organisms?
Answers
Answer:
a Binomial Naming System
Explanation:
HELP PLEASE!
Based on your Retention factor values for the 5 dye standards, order the dyes from most polar to least polar.
Red #3 - 0.000
Blue #1 - 0.58
Yellow #5- 0.286
Red #40- 0.046
Blue #2- 0.095
Answers
Answer:
Red #3 is the most polar, and Blue #1 is the least polar.
Explanation:
To order the dyes from most polar to least polar, we need to look at the retention factor values. The lower the retention factor value, the more polar the molecule. So, based on the given values, the order from most polar to least polar would be:
Red #3 - 0.000
Yellow #5 - 0.286
Blue #2 - 0.095
Red #40 - 0.046
Blue #1 - 0.58
what does rows represent on the periodic table
Answers
Answer:
Rows on the periodic table represents a period, which is the number of energy levels or rings. It is the last shell which valence electrons are on.
For example the first row waas Hydrogen and Helium which each have one energy level.
The esecond row has Carbon and Oxygen which has two energy level.
The third row has Sodium which has three energy levels and so on.
What is The relationship between the electromotive force and the free enthalpy of reaction in a redox reaction?
Answers
Answer:
The EMF and free enthalpy (or Gibbs Free Energy) of a reaction are directly related.
If the free enthalpy of a redox reaction is negative, then the EMF will be negative, indicating that the reaction is spontaneous and will occur without the need for an external source of energy.
If the free enthalpy of the reaction is positive, then the EMF will be positive, indicating that the reaction is not spontaneous and will not occur without the input of energy.
The relationship between free enthalpy and EMF is shown in the following equation:
∆G = -nFE°(cell), where n is the number of electrons transferred, F is the Faraday constant, and E° is the EMF of a cell under standard conditions.
Carbon dioxide is a compound of the elements carbon and oxygen (CO2). What happened to the carbon and oxygen to create this compound?
A. they merged nuclei
B. they swapped protons
C. they combined neutrons
D. they exchanged electrons
Answers
Answer:
D. they exchanged electrons
"The sun was setting behind the mountain when Isaiah reached the first fields of crops, bordered by low white stone walls. At the end of the road was the village, built on a slope, whose houses sank with all their weight into the ground, as if in fear of sliding further down. Roofs of overlapping slates peered down on the tiny, lifeless windows. The tall wooden chimneys, shaped like truncated pyramids, smoked quietly in the evening. This place was the extreme point where men had dared to plant a shelter and sow the grain. But, on the rough, rocky soil, even the rye did not grow well. The old men died without having saved anything, and the young, one after the other, fled from this corner of bad earth that the snowfalls isolated from the world for six months of the year. Once prosperous and populated to the rim, the village now had barely eighteen fires. And above it there were only shelters lost in the mountains for summer climbers. "
1.This description allows us to identify the village of the two brothers as being mainly
a. An isolated high mountain village in which the inhabitants work hard and see their efforts rewarded.
b. A quiet high-altitude village in which many courageous young farmers live.
c. A village in total harmony with the wilderness that still can't feed its children.
d. A pleasant village in the heart of the rugged mountains that welcomes many climbers in summer.
Answers
░░░░░▐▀█▀▌░░░░▀█▄░░░
░░░░░▐█▄█▌░░░░░░▀█▄░░
░░░░░░▀▄▀░░░▄▄▄▄▄▀▀░░
░░░░▄▄▄██▀▀▀▀░░░░░░░
░░░█▀▄▄▄█░▀▀░░
░░░▌░▄▄▄▐▌▀▀▀░░
▄░▐░░░▄▄░█░▀▀ ░░
▀█▌░░░▄░▀█▀░▀ ░░
░░░░░░░▄▄▐▌▄▄░░░
░░░░░░░▀███▀█░▄░
░░░░░░▐▌▀▄▀▄▀▐▄░░
░░░░░░▐▀░░░░░░▐▌░░
░░░░░░█░░░░░░░░█░
This is bob. If you brainliest him. He will reward you with a certain script.
The pH of a solution is 2.0. Which statement is correct? Useful formulas include , , , and .
Answers
Answer:
if the pH of a solution is 2.0 that means the solution would be acidic.
Explanation:
the ___is the area that allows us to pinpoint the location of pain, identify a texture and be aware of how our limbs are positioned?
Answers
Answer:
The somatosensory cortex is a region of the brain which is responsible for receiving and processing sensory information from across the body, such as touch, temperature, and pain.
Area 1 specifically is important in sensing the texture of an object. :)
Hope this helps!
Using a triple beam balance and a graduated cylinder, a student collected data on a sample of an element:Mass of sample = 18.9 gVolume of water = 30.0 mLVolume of water and sample = 35.0 mL..... using SigFig Rules *
Answers
The question is incomplete; the complete question is;
Using a triple beam balance and a graduated cylinder, a student collected data on a sample of an element:
Mass of sample = 18.9 g
Volume of water = 30.0 mL
Volume of water and sample = 35.0 mL
Calculate the density of the sample using sig figs.
Answer:
3.8 g/mL
Explanation:
Mass of sample = 18.9 g
Volume of water = 30.0 mL
Volume of water and sample = 35.0 mL
Volume of the sample = 35.0 mL – 30.0 mL = 5.0 mL
D = m/v
D = 18.9 g / 5.0 mL =3.8 g/mL
Diagrams below represent four states of matter. which is ionized?
Answers
The diagram representing the ionized state of matter is the plasma state.
2Diagrams of four states of matter
Solid: A solid is a state of matter in which the constituent particles, such as atoms, molecules, or ions, are packed together tightly and firmly held by strong intermolecular forces.
Liquid: A liquid is a state of matter in which the constituent particles, such as atoms, molecules, or ions, are closely packed together but are not held together as strongly as in solids.
Gas: A gas is a state of matter in which the constituent particles, such as atoms, molecules, or ions, are widely separated and are not held together as strongly as in liquids and solids.
Plasma: Plasma is a state of matter in which a gas has been ionized, and it becomes a collection of charged particles such as positive ions and free electrons.
Plasma is a type of ionized gas in which the gas is composed of ions, electrons, and neutral atoms.
Plasma is a state of matter that is similar to a gas in that it has no fixed shape or volume, but unlike gas, it is made up of electrically charged particles.
The electrical charges in plasma are due to the presence of both positive and negative ions.
The most common example of plasma is lightning, and it is also present in fluorescent lights, neon signs, and plasma TVs.
For more such questions on plasma state
https://brainly.com/question/5055528
#SPJ8
what is the mass number of an atom or ion with one proton, two neutrons, and one electron?
Answers
Answer:
3
Explanation:
mass no.=protons+neutrons
mass no.=1+2=3
Assuming that protein synthesis was under way when the radioactive amino acids were added, which of the following best describes how the radioactivity was distributed in one of the first molecules of Protein X that was completely translated?
A.Radioactive amino acids were randomly located throughout the molecule.
B.Radioactive amino acids were located only at one end of the molecule.
C.Radioactive amino acids were located at both ends, but not in the middle, of the molecule.
D.Radioactive amino acids were located in the middle, but not at either end, of the molecule.
Answers
The statement that best describes how the radioactivity was distributed in one of the first molecules of Protein X that was completely translated is B. Radioactive amino acids were located only at one end of the molecule.
Protein X was one of the first molecules to be completely translated, and the distribution of its radioactivity was unique. Radioactive amino acids were not dispersed throughout the molecule, but instead were located only at one end of the molecule.
This means that the statement B best describes how the radioactivity was distributed in one of the first molecules of Protein X that was completely translated.
With this knowledge, we can be sure that the radioactivity was not evenly distributed throughout the molecule, but was instead concentrated at one end.
This information provides us with a better understanding of the structure of Protein X, and how it is different from other molecules.
To learn more about amino acids, click here:
https://brainly.com/question/28362783
#SPJ4
Name a liquid substance that could be used in the laboratory for: dissolving dry mortar on floor tiles; (i) removing KMnO, stains; drying acid anhydrides
Answers
A liquid substance that could be used in the laboratory for dissolving dry mortar on floor tiles is vinegar; (i) removing KMnO₄, stains is sodium metabisulfite solution; drying acid anhydrides is concentrated sulfuric acid.
What are solvents?Solvents are substances usually liquids, but may also be gases or solids that dissolve other substances known as solutes.
Solvents are usually used as cleansing agents.
One possible liquid substance that could be used for dissolving dry mortar on floor tiles is a mild acid solution, such as diluted hydrochloric acid or vinegar.
KMnO₄ stains are often difficult to remove, but one substance that can be used is sodium metabisulfite (Na₂S₂O₅) solution. Sodium metabisulfite acts as a reducing agent and can effectively neutralize and remove KMnO₄ stains.
Concentrated sulfuric acid is commonly used in the laboratory as a drying agent. It has a strong affinity for water and can efficiently absorb moisture, including water present in acid anhydrides.
Learn more about solvents at: https://brainly.com/question/25326161
#SPJ1