Answers
no se bb si quieres salimos tu sabes
Related Questions
Please help me solve this

Answers
1. No the shape will remain the same, as no matter the type of bond, triple, single, or double the shape will remain, it only changes if a bond is added.
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8
Please if you know the answer put it thanks

Answers
The diagram shows a picture of a compound.
What is a compound?A compound is a substance that is made up of two or more different elements that are chemically bonded together in a specific ratio.
This means that the elements are combined in a way that creates a new substance with different physical and chemical properties than the individual elements.
Compounds can be formed through a variety of chemical reactions, such as combining elements through a chemical bond or through a reaction between an acid and a base.
So for the given diagram, we can see that it represents two or more elements chemically combined.
Learn more about a compound here: https://brainly.com/question/29108029
#SPJ1
Water is a polar solvent and hexane ( (C 6H 14) s a nonpolar solvent. Which of the following correctly describes the solubility of the solute? o octane (nonpolar), soluble in water O Cacl2, soluble in hexane O CCl4 (nonpolar), soluble in water O mineral oil (nonpolar), soluble in water O NaHCO3, soluble in water
Answers
NaHCO3 is soluble in water. NaHCO3 is ionic polar compound. water is polar quantities so, polar disolve in polar.
The basic concept of solubility is that like dissolves like. It means that polar substances are soluble in polar solvents and non-polar substances are soluble in non-polar solvents.
Solubility is described as the most amount of a substance so that it will dissolve in a given amount of solvent at a exact temperature. Solubility is a function property of a particular solute–solvent aggregate, and unique substances have substantially differing solubilities.
Solubility is the ability of a substance (solute) to form a solution with another substance (solvent). Insolubility is the opposite property, the inability of a solute to form such a solution.
Learn more about solubility here:
https://brainly.com/question/23946616
#SPJ4
Question 9
Charles runs two laps around 500 m track. Waht is his total distance traveled?
distance traveled =
m
Answers
Answer:
1000 or 250
Explanation:
500x2=1000
or
500/2=250
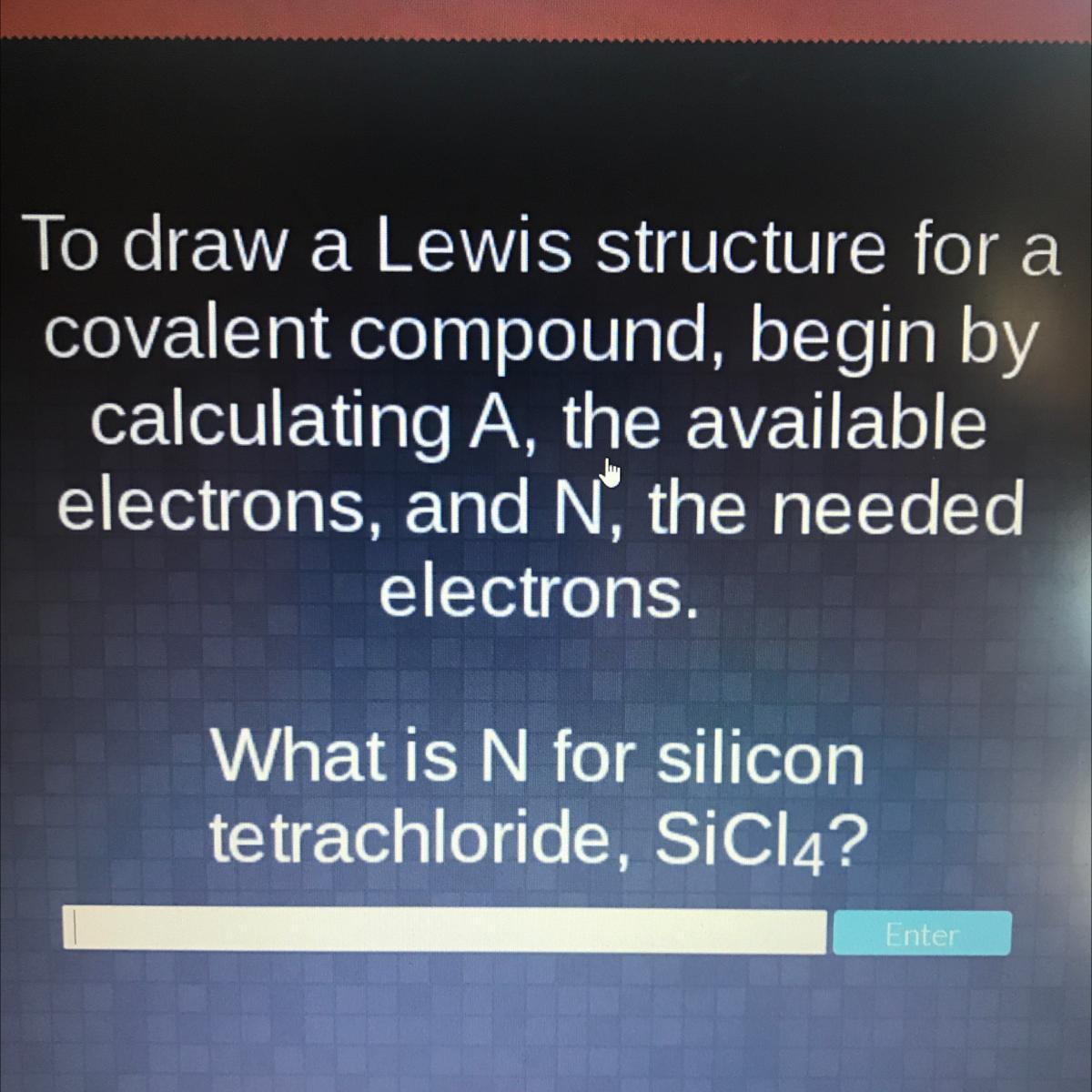
What is N for silicon tetrachloride, SiCl4?
Answers
PLEASE HELP ME! CHEMISTRY!
reactants: calcium chloride + sodium bicarbonate
products: carbon dioxide + calcium carbonate + sodium chloride + dihydrogen monoxide
question; is this equation balanced? explain reason. if not balanced, how should it be balanced? what is the balanced equation?
Answers
Answer:
No it is not balanced
Explanation:
CaCl2 + 2NaHCO3 → CO2 + CaCO3 + H2O + 2NaCl
Hope this helps
Amy set up the following experiment to study how plant growth is influenced by weather conditions. mc052-1 One pot will model normal growing conditions, and the other will model drought conditions. Which of the following factors is it most important for Amy to change to model drought conditions?
Answers
Answer:
lack of sunlight, overcrowded region for shelter of the plants, no nutrients and water supply, etc.
Explanation:
The plants needs favorable climatic conditions and nutrient to grow. Plants need air, sunlight, water, proper temperature, nutrients, etc. grow and make their food. They make their food with the help of sunlight and carbon dioxide by a process called as photosynthesis.
Now in the context, Amy is setting up an experiment where she will study the growth of plants in two models. One in the normal growing conditions and the other in a drought condition.
Drought is considered the most important abiotic factor that limits the growth and adversely affects the growth and the crop. The factor that is important for Amy to change the model into a drought conditions is the lack of water and carbon dioxide in the atmosphere. Also scarcity of sunlight and nutrients. The drought stress is considered as one of the crop performance limiting factors.
7 of 207 of 20 Items
10:54
Question
Most foxes are brown or orange in color. However, foxes are light silver or nearly white in the far northern tundra. What is a MOST LIKELY reason for this color difference?
Responses
A Arctic foxes have developed teeth that allow them to eat frozen carrion.Arctic foxes have developed teeth that allow them to eat frozen carrion.
B Arctic foxes have developed coloring based on their nonliving environment.Arctic foxes have developed coloring based on their nonliving environment.
C Arctic foxes have developed hunting habits that helps them find food.Arctic foxes have developed hunting habits that helps them find food.
D Arctic foxes have developed special eye coverings that let them see even during blizzards.
Answers
Answer:
B
Explanation:
Most animals change due to the environment to help them live better
I need help on this organic chemistry question:
------
What are the number of rotational axes and the number of mirror planes for each of these images? (below)
1,1-dichlorocyclopropane
trans-1,2-dichlorocyclopropane
cis-1,2-dichlorocyclopropane
cis-1-bromo-2,3-dichlorocyclopropane (all cis)
1-bromo-1-chlorocyclopropane
trans-1-bromo-2-chlorocyclopropane
cis-1-bromo-2-chlorocyclopropane
Which of these are superimposable?
-------
Thank you!! :)
Answers
Sodium carbonate, also known as soda ash, is used in glassmaking. It is obtained from a reaction between sodium chloride and calcium carbonate; calcium chloride is the other product. Calculate the percent yield of sodium carbonate if 92.6 g is collected when 112. g of sodium chloride reacts with excess calcium carbonate.
Answers
Answer:
The percentage yield of sodium carbonate is 91.47%
Explanation:
we start by writing the reaction equation:
2NaCl + CaCO3 ——-> Na2CO3 + CaCl2
From the reaction we can see that 2 moles of sodium chloride produced 1 mole of sodium carbonate
Let us calculate the actual number of moles of sodium chloride produced from 112 g of it
Mathematically,
number of moles = mass/molar mass
Molar mass of sodium chloride is 23 + 35.5 = 58.5 g/mole
So the number of moles of sodium chloride produced will be 112/58.5 = 1.91 moles
The number of moles of sodium carbonate produced is half of this = 1.91/2 = 0.955
The mass of sodium carbonate produced from 0.955 moles of it will be;
number of moles * molar mass
The molar mass of sodium carbonate is 106 g/mol
So the number of moles is = 0.955 * 106 = 101.23 g
Mathematically;
percentage yield = actual yield/theoretical yield * 100%
Percentage yield = 92.6/101.23 * 100% = 91.47%
all first level consumers are carnivore: True or false?
Answers
The fluorocarbon compound C2Cl3F3 has a normal boiling point of 47.6 ∘C. The specific heats of C2Cl3F3(l) and C2Cl3F3(g) are 0.91 J/g⋅K and 0.67 J/g⋅K, respectively. The heat of vaporization for the compound is 27.49 kJ/mol.
Part A
Calculate the heat required to convert 75.0 g of C2Cl3F3 from a liquid at 13.60 ∘C to a gas at 76.00 ∘C.
Answers
The heat required is to convert 75.0 g of C₂Cl₃F₃ from a liquid at 13.60 ∘C to a gas at 76.00 ∘C is 17.55 kJ.
To solve this problem, we need to consider the different steps involved in the process of converting 75.0 g of C₂Cl₃F₃ from a liquid at 13.60 ∘C to a gas at 76.00 ∘C;
Heating the liquid C₂Cl₃F₃ from 13.60 ∘C to its boiling point at 47.6 ∘C, Vaporizing the liquid C₂Cl₃F₃ at its boiling point, and heating the resulting gas from 47.6 ∘C to 76.00 ∘C
Now, we can use the equations to calculate the heat required for each step;
q₁ = m × C₁ × ΔT₁
where q₁ is the heat required, m is the mass of C₂Cl₃F₃, C₁ is the specific heat of C₂Cl₃F₃(l), and ΔT₁ is the temperature change from 13.60 ∘C to 47.6 ∘C.
q₁ = 75.0 g × 0.91 J/g⋅K × (47.6 ∘C - 13.60 ∘C)
= 2466 J
q₂ = n × ΔHvap
where q₂ is the heat required, n is the number of moles of C₂Cl₃F₃, and ΔHvap is the heat of vaporization of C₂Cl₃F₃.
n = m/M
= 75.0 g / 137.37 g/mol
= 0.5464 mol
q₂ = 0.5464 mol × 27.49 kJ/mol
= 15.038 kJ
q₃ = m × C₂ × ΔT₂
where q₃ is the heat required, m is the mass of C₂Cl₃F₃(g), C₂ is the specific heat of C₂Cl₃F₃(g), and ΔT₂ is the temperature change from 47.6 ∘C to 76.00 ∘C.
m = n × M
= 0.5464 mol × 137.37 g/mol
= 75.0 g
q₃ = 75.0 g × 0.67 J/g⋅K × (76.00 ∘C - 47.6 ∘C)
= 1446 J
The total heat required is the sum of the heats required for each step;
\(q_{total}\)= q₁ + q₂ + q₃
= 2466 J + 15.038 kJ + 1446 J
= 17.55 kJ
Therefore, the total heat required is 17.55 kJ.
To know more about heat required here
https://brainly.com/question/14679329
#SPJ1
Write an equation that represents the action in water of calcium hydroxide as an Arrhenius base
Answers
Explanation:
Calcium hydroxide = Ca(OH)₂
Water = H₂O
An Arrhenius base is a substance which interacts with water to yield excess hydroxide ions, OH⁻ in an aqueous solution.
The reaction in water;
Ca(OH)₂ → Ca²⁺ + 2OH⁻
The medium of this reaction is water.
URGENT PLZ HELP
According to AUFBAU, which of the following sublevels is of highest energy?
a) 2p
b) 3d
c) 3s
d) 4s
Answers
2. 20.0 mL of a 0.75 M solution
of potassium permanganate,
KMnO solution is used to
make a 250.00 mL solution.
What is the concentration of
the new solution?
Answers
The concentration of the new potassium permanganate, KMnO solution is 0.06 M.
To find the concentration of the new solution, we can use the formula,
C₁V₁ = C₂V₂,
C₁ = 0.75,
V₁ = 20.0 mL,
V₂ = 250.0mL
C₂ is what we have to find. Plugging in the values we know, we get,
0.75 M x 20.0 mL = C₂ x 250.00 mL
Solving for C₂, we get the final concentration of the potassium permanganate.
C₂ = (0.75 M x 20.0 mL) / 250.00 mL
C₂ = 0.06 M
Therefore, the concentration of the solution has changed to 0.06 M from 0.75M.
To know more about concentration, visit,
https://brainly.com/question/17206790
#SPJ1
Which of the following set of quantum numbers (ordered n, ℓ, mℓ ) are possible for an electron in an atom? Check all that apply
a. 2, 1, 3
b. 5, 3, -3
c. 4, 3, -2
d. -4, 3, 1
e. 2, 1, -2
f. 3, 2, 2
g. 3, 3, 1
Answers
the possible quantum numbers (ordered n, ℓ, mℓ ) are:Option B.5, 3, -3 and Option C. 4, 3, -2
The quantum numbers n, ℓ, mℓ represent respectively the principal quantum number, the orbital angular momentum quantum number and the magnetic quantum number.
These are the three most important quantum numbers. T
here is another quantum number called the spin quantum number, denoted by ms.
Let's see which of the given quantum number sets is possible.2, 1, 3 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. 5, 3, -3 is possible.4, 3, -2 is possible. -4, 3, 1 is not possible.
For any value of ℓ, mℓ must be between -ℓ and +ℓ. e. 2, 1, -2 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. f. 3, 2, 2 is not possible because for ℓ = 2, mℓ can only be -2, -1, 0, +1, or +2. g. 3, 3, 1 is not possible because for any value of ℓ, mℓ must be between -ℓ and +ℓ.
Therefore, the possible quantum numbers (ordered n, ℓ, mℓ ) are:5, 3, -34, 3, -2
For more questions on quantum numbers
https://brainly.com/question/30881398
#SPJ8
How many milligrams are in 30.23 pounds? Do not use units. Answer in standard notation using the correct number of significant figures.
Answers
Answer:
1371 would be the right calculation
if a molecule contains polar bonds the molecule __ must be polar overall
Answers
Answer:
diatomic
Explanation:
D » » DI
Which applies to fusion? Check all that apply.
involves the splitting of nuclei
takes place in the Sun
releaſes radiation as a waste product
occurs in nuclear power plants and is used to generate electricity
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
I’m looking for fusion not fission
Answers
Answer:
The correct answer is -
takes place in the Sun
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
Explanation:
Fusion reaction takes place when two or more small atomic nuclei come in close proximation for a longer time so the nuclear force pulling them together and form into heavier molecules than helium and releases a huge amount of energy by this process.
A great example of this fusion reaction is the sun where nuclear fusion takes place inside the core of the sun and result in a huge amount of release as it is an exothermic reaction.
Answer:
2, 5, 6Explanation
EDGE2021
What is the hardest things in the world
Answers
Answer:
Diamond is the hardest things in the world
The density of chlorine gas at 0.970 atm and 29.8 °C is ________ g/L.
Answers
Answer:
d=3.95 g/l
Explanation:
1) Ideal gas equation:
pV = nRT
2) Transform the equation to compute density, d = m / V
n = mass in grams / molar mass = m / MM
pV = (m/MM) RT
=> pV = mRT/MM
=> m/V = pMM / (RT)
d = pMM / (RT)
p = 1.21 atm
MM = 83.80 g/mol (this is the atomic mass of krypton element)
T = 50 + 273.15 K = 323.15K
3) Compute:
d = (1.25 atm * 83.80 g/mol) / (0.0821 atm*liter /K*mol * 323.15K)
Calculate the mass percent composition of each constituent element of lead (II) phosphate
Pb =
P =
O =
Answers
The mass percent composition of each constituent element of lead (II) phosphate is:
Pb = 76.58%
P = 7.63%
O = 15.79%
To calculate the mass percent composition of each constituent element of lead (II) phosphate, we need to first find the molar mass of each element in the compound and then divide each by the total molar mass of the compound.The chemical formula for lead (II) phosphate is Pb3(PO4)2.
The molar mass of each element in the compound is:
Pb = 207.2 g/mol
P = 30.97 g/mol
O = 16.00 g/mol
The total molar mass of the compound is:
Pb3(PO4)2 = (3 × 207.2) + (2 × 30.97) + (8 × 16.00) = 811.34 g/mol
The mass percent composition of each element is:
Pb = (3 × 207.2) / 811.34 × 100 = 76.58%
P = (2 × 30.97) / 811.34 × 100 = 7.63%
O = (8 × 16.00) / 811.34 × 100 = 15.79%
More questions on mass percent can be obtained here: https://brainly.com/question/23896069
#SPJ11
Determine the energy release in the reaction13 13 0N ----> C + e7 6 +1Calculate (a) using nuclear masses, formed by subtracting the proper number of electron masses from the atomic masses, and (b) using atomic masses while accounting for the energy of pair production.
Answers
The energy release of the reaction is 4.852 x 1017 J using nuclear masses, and 3.972 x 1017 J using atomic masses while accounting for the energy of pair production.
To calculate the energy release in this reaction, we can use the following equation:
Energy release = (Mass of Reactants - Mass of Products) x c2
(a) Using nuclear masses:
Mass of Reactants = 13.001354 u
Mass of Products = 7.016004 u + 1.008665 u = 8.024670 u
Energy Release = (13.001354 - 8.024670) x 9 x 1016 = 4.852 x 1017 J
(b) Using atomic masses while accounting for the energy of pair production:
Mass of Reactants = 13.001354 u
Mass of Products = 7.016004 u + 1.008665 u = 8.024670 u
Energy Release = (13.001354 - 8.024670 - 2(0.510998928) ) x 9 x 1016 = 3.972 x 1017 J
Therefore, the energy release of the reaction is 4.852 x 1017 J using nuclear masses, and 3.972 x 1017 J using atomic masses while accounting for the energy of pair production.
Know more about nuclear masses
https://brainly.com/question/26806573
#SPJ11
Calculate the molarity of 0.300 mol of Na₂S in 1.15 L of solution.
molarity:
Calculate the molarity of 34.7 g of MgS in 935 mL of solution.
molarity:
Answers
Answer:
1) 0.261 M, 2) 0.662 M
Explanation:
1. We must calculate the molarity of 0.300 mol of Na₂S in 1.15 L of solution.
We know, Molarity = moles of solute/ volume of solution
= 0.300 mol/ 1.15 L
= 0.261 M
2. We must calculate the molarity of 34.7 g of MgS (56 u) in 935 mL of solution.
We know, Molarity = moles of solute/ volume of solution
Moles of MgS = 34.7/56 = 0.619
Volume of solution = 935 mL = 0.935 L
= 0.619 mol/ 0.935 L
= 0.662 M
How does using more water in a beaker affect the solubility in an experiment?
Answers
Answer:
it may lead to read false measurements
while in an experiment.
that's what I think.
Describe how crystals change if they form in large amounts of space vs limited space.
Answers
Crystals form when magma cools because the solution is super-saturated with certain minerals. Because the crystals do not have much time to form if the magma cools quickly, they are very small. The crystals have enough time to grow and become large if the magma cools slowly.
What causes big crystals to form?When the solution becomes supersaturated, which means there is too much salt dissolved in the water, crystals form. Crystals form from the extra salt (or other material). To obtain a supersaturated solution, either cool the solution or allow some of the water to evaporate.
The size of the crystals is affected by the solubility of the compound in the solvent used for recrystallization, the number of nucleation sites, mechanical agitation of the system, and time during crystal growth.
Thus, the crystals do not have much time to form if the magma cools quickly, they are very small.
To learn more about the crystals, follow the link;
https://brainly.com/question/13008800
#SPJ9
Electrochemical cells generate electricity from which of the following? Select all that apply.
electron transfer
flow of electrons
dissolving an ionic compound
redox reactions
Answers
By a redox reaction that involves the transfer of electrons, often through the dissolution of an ionic substance, electrochemical cells produce electricity from the flow of electrons.
What fuels the production of energy by electrochemical cells?In electrochemistry, redox or oxidation-reduction reactions, in which electrons travel from one element to another, can produce electricity. Redox processes involve the transfer of electrons from one substance to another.
In what element are electrochemical cells made?Batteries use a very significant class of oxidation and reduction reactions to produce useable electrical energy. Using solutions of respective sulphates, copper and zinc metals can be combined to create a straightforward electrochemical cell.
To know more about electrons visit:-
https://brainly.com/question/20513633
#SPJ1
A gas has a pressure of 2.70 atm at 50.0 °C. What is the pressure at standard temperature (0°C)?
Answers
Answer:
2.282 atm
P1V1/T1 = P2V2/T2
2.70atm / (50+273) = X/ 273
make x subject of formula
:. X = 2.28 atm
or 2.28 * 1.01 *10⁵ N/m²
you can support by rating brainly it's very much appreciated ✅✅
2. A sample containing 1.80 mol of argon gas has a volume of 10.00 L. What is
the new volume of the gas, in litres, when each of the following changes occurs in
the quantity of the gas? Assume that pressure and temperature remain constant.
The changes are not cumulative. T
(a) An additional 1.80 mol of argon gas is added to the container. [ans: 20.0 L]
(b) A sample of 25.0 g of argon gas is added to the container. [ans: 13.5 LJ
(c) A hole in the container allows half of the gas to escape. [ans: 5.00 L]
3. A balloon that contains 4.80 g of carbon dioxide gas has a volume of 12.0 L. Assume
that the pressure and temperature of the balloon remain constant. What is the new
volume of the balloon if an additional 0.50 mol of CO₂ is added? [ans: 67 L]
Answers
Answer:
Hi goodmorning
The total volume of the gas is then 12.0 L + 0.806 L = 12.806 L.
To answer these questions, you can use the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas. The ideal gas law is given by the following equation:
PV = nRT
Where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature of the gas.
If pressure and temperature remain constant, then the volume of the gas will change inversely with the number of moles of the gas. This means that if the number of moles of the gas increases, the volume of the gas will decrease, and if the number of moles of the gas decreases, the volume of the gas will increase.
For example, in part (a), the number of moles of argon gas in the container increases by 1.80 mol, so the volume of the gas will decrease. The new volume of the gas can be calculated as follows:
V = (nRT)/P
= (1.80 mol * 8.31 J/mol*K * 300 K)/(1 atm)
= 4452 J
= 4.452 L
The total volume of the gas is then 10.00 L + 4.452 L = 14.452 L.
In part (b), the number of moles of argon gas in the container increases by a certain amount, which you can calculate using the molar mass of argon. The molar mass of argon is 39.948 g/mol, so the number of moles of argon in 25.0 g of argon is 25.0 g / 39.948 g/mol = 0.625 mol. The new volume of the gas can be calculated as follows:
V = (nRT)/P
= (0.625 mol * 8.31 J/mol*K * 300 K)/(1 atm)
= 1478.125 J
= 1.478 L
The total volume of the gas is then 10.00 L + 1.478 L = 11.478 L.
In part (c), the number of moles of argon gas in the container decreases by half, so the volume of the gas will increase. The new volume of the gas can be calculated as follows:
V = (nRT)/P
= (0.9 mol * 8.31 J/mol*K * 300 K)/(1 atm)
= 2463.9 J
= 2.464 L
The total volume of the gas is then 10.00 L + 2.464 L = 12.464 L.
In part (d), the number of moles of CO2 gas in the balloon increases by 0.50 mol, so the volume of the gas will decrease. The molar mass of CO2 is 44.01 g/mol, so the number of moles of CO2 in 4.80 g of CO2 is 4.80 g / 44.01 g/mol = 0.109 mol. The new volume of the balloon can be calculated as follows:
V = (nRT)/P
= (0.109 mol + 0.50 mol) * 8.31 J/mol*K * 300 K)/(1 atm)
= 806.36 J
= 0.806 L