Changing gloves often will minimize the spread of chemicals. To properly remove gloves, first, Choose... , and then, Choose... . Then insert Choose... between the interior edge of the second glove and the skin, and remove the second glove.
Answers
Peel the glove away from your body, pulling it inside out. Peel the glove away from your body, Grasp the outside of one glove at the wrist. Do not touch your bare skin.
What are chemicals?A chemical is any substance that has a defined composition.
Steps to remove gloves:
Grasp the palm of one glove near your wrist. Carefully pull the glove off, turning it inside out.
Hold the glove in the palm of the still-gloved hand. ...
Pull the glove until it comes off inside out. ...
Always wash your hands after removing gloves and before touching any objects or surfaces.
Learn more about the chemicals here:
https://brainly.com/question/13145357
#SPJ1
Related Questions
Given the following half-reactions and their respective standard reduction potentials 1.) Cu3+ + 2e- ---> Cu+....E1= 1.128V 2.) Cu2+ + e- ---> Cu+....E2=0.15V 3.) Cu2+ + 2e- ---> Cu(s)....E3=0.34V 4.) Cu3+ + 2e- ---> Cu+....E4=0.52V calculate the standard reduction potential for the reduction half-reaction of Cu(III) to Cu(II). Cu3+ + e- ---> Cu2+ E= ? V
Answers
The standard reduction potential for the reduction half-reaction of Cu(III) to Cu(II) is 0.608V.
E = 1.128V - 0.52V = 0.608V
The standard reduction potential for the reduction half-reaction of Cu(III) to Cu(II) can be calculated by subtracting the standard reduction potential for the reaction Cu3+ + 2e- ---> Cu+ (E4) from the standard reduction potential for the reaction Cu3+ + 2e- ---> Cu(s) (E3). Thus, the standard reduction potential for the reduction half-reaction of Cu(III) to Cu(II) is E = 1.128V - 0.52V = 0.608V.
The standard reduction potential for the reduction half-reaction of Cu(III) to Cu(II) is 0.608V.
learn more about standard reduction potential here
https://brainly.com/question/23881200
#SPJ4
calculate the molar solubility of ca(oh)2 in 0.10 m ca(no3)2 (ksp= 1.3x10^-6) in pure water
Answers
Ca(OH)₂⇒ Ca²⁺ + 2OH⁻
s s 2s
Ksp = [Ca²⁺][OH⁻]²
Ca(NO₃)₂ ⇒ Ca²⁺ + 2NO₃⁻
0.1 M 0.1 0.2
Input in Ksp
1.3 x 10⁻⁶ = 0.1 . 4s²
s² = 3.25 x 10⁻⁶
s = 1.8 x 10⁻³
1.8 x 10⁻³ is the molar solubility. Solubility is the amount of a substance that can be dissolved in a liquid to form a solution.
What is solubility?Solubility is the amount of a substance that can be dissolved in a liquid to form a solution; it is typically represented as grammes of solute every litre of liquid. One fluid's (liquid or gas) solubility in another can be entire (e.g., methanol and water are completely miscible) or partial (e.g., oil and water barely mix). Generally speaking, "like dissolves like" (for instance, the aromatic hydrocarbons dissolves in one another but not in water).
A material's solubility in two solvents is measured by the distribution coefficient, which is used in some separation techniques (such as absorption and extraction). In general, as temperature rises, so do the dissolution rates of solids in liquids, while they fall as temperature rises and rise with pressure for gases.
Ca(OH)₂⇒ Ca²⁺ + 2OH⁻
s s 2s
Ksp = [Ca²⁺][OH⁻]²
Ca(NO₃)₂ ⇒ Ca²⁺ + 2NO₃⁻
0.1 M 0.1 0.2
1.3 x 10⁻⁶ = 0.1 . 4s²
s² = 3.25 x 10⁻⁶
s = 1.8 x 10⁻³
Therefore, 1.8 x 10⁻³ is the molar solubility.
To know more about solubility, here:
https://brainly.com/question/14366471
#SPJ2
If a molecule has bond angles of 120° between the atoms, what type of hybrid orbitals are on the central atom in the molecule?
Answers
Answer:
Solution
verified
Verified by Toppr
Correct option is B)
In sp
3
d type of hybridisation, shape of molecule is trigonal bipyramidal and bond angle will be of 120
o
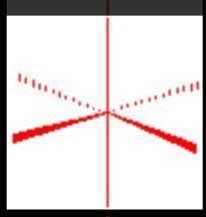
A cancer laboratory is estimating the rate of tumorigenesis in two strains of mice, A and B, denoted θ A
and θ B
, respectively. They have tumor count data for 10 mice in strain A and 13 mice in strain B. Type A mice have been well-studied, and information from other laboratories suggests that type A mice have tumor counts that are approximatelyPoisson distributed with a mean of 12 . Tumor count rates for type B mice are unknown, but type B mice are related to type A mice. The observed tumor counts for the two populations are: y A
=(12,9,12,14,13,13,15,8,15,6)
y B
=(11,11,10,9,9,8,7,10,6,8,8,9,7).
(a) (2 points) Assume the following prior distributions for the two types of mice: θ A
∼ Gamma(120,10) and θ B
∼Gamma(12,1). Plot the priors in R, and compare them. Explain how these distributions adequately depict the prior information available to the laboratory. (b) (2 points) Assume that θ A
and θ B
are independent of each other. Identify the posterior distributions for θ A
and θ B
(using derived results from class). Plot the posteriors in R, and compare them. (c) (2 points) Find the posterior mean, variance, and maximum a posteriori (MAP) estimates for θ A
and θ B
using known formulas. (d) (4 points) Use R to find 99% percentile-based and highest density region credible intervals for θ A
and θ B
. Why do the two credible interval procedures produce similar results? (Hint: use the HDInterval R package) (e) (4 points) Compute and plot the posterior expectation of θ B
under the prior distribution θ B
∼Gamma(12n 0
,n 0
) for each value of n 0
∈{1,2,…,50}. Describe what sort of prior beliefs about θ B
would be necessary in order for the posterior expectation of θ B
to be close to that of θ A
. (f) (2 points) In part (a), we assumed θ A
and θ B
are independent. Does this assumption make sense in this study? Explain your answer.
Previous question
Answers
Compute and plot the posterior expectation of θB under different prior beliefs.
Plot the prior distributions for θA and θB and explain their representation of prior information?The prior distributions for θA and θB are Gamma(120, 10) and Gamma(12, 1), respectively. Plotting these priors in R allows us to visualize their shapes and compare them.
The Gamma distribution is a flexible family of continuous probability distributions that is suitable for modeling positive-valued variables like tumor count rates.
The choice of parameters for the priors reflects prior knowledge about the mean and variability of tumor counts in each strain. Gamma(120, 10) indicates a higher mean and lower variability for strain A compared to Gamma(12, 1) for strain B. The prior distributions effectively capture the available prior information, allowing for different expectations and levels of uncertainty for the two strains.
Assuming independence between θA and θB, we can calculate the posterior distributions using the derived results from class.
The posterior distributions for θA and θB will also be Gamma distributions. The posterior parameters can be computed by adding the observed tumor counts to the prior parameters. Plotting the posteriors in R allows us to visualize the updated beliefs about the tumor count rates in each strain based on the observed data.
Learn more about prior
brainly.com/question/30300617
#SPJ11
The watt is the standard unit of measurement for?
Answers
Answer:
electrical power. nnnnnnn
Name these compounds according to IUPAC. (ASAPP)

Answers
Propanal and acetone are the IUPAC names for the chemical.
What is the difference between propanal and acetones?Propanal, commonly known as propanaldehyde, is an aldehyde with one double bond and one oxygen atom.
Acetone is a ketone compound. R2CO is the functional group of ketones.
Thus, Propanal and acetone are the IUPAC names for the chemical.
Learn more about propanal and acetones
brainly.com/question/25702257
#SPJ1
What are 10 examples of homogeneous mixtures?
Answers
A homogeneous mixture is a mixture where the components are uniformly distributed throughout the mixture, resulting in a uniform composition and appearance. Here are 10 examples of homogeneous mixtures:
Saltwater: When salt is dissolved in water, the resulting mixture is homogeneous, with the salt molecules evenly distributed throughout the water.
Air: The air we breathe is a mixture of gases, including nitrogen, oxygen, and carbon dioxide. These gases are evenly distributed throughout the atmosphere, resulting in a homogeneous mixture.
Sugar solution: When sugar is dissolved in water, the resulting mixture is homogeneous, with the sugar molecules evenly distributed throughout the water.
Vinegar: Vinegar is a homogeneous mixture of acetic acid and water, with the two components evenly distributed throughout the solution.
Milk: Milk is a homogeneous mixture of water, fat, protein, and sugar, with these components evenly distributed throughout the liquid.
Brass: Brass is a homogeneous mixture of copper and zinc, with the two metals evenly distributed throughout the alloy.
Gasoline: Gasoline is a homogeneous mixture of hydrocarbons and other additives, with the components evenly distributed throughout the liquid.
Blood: Blood is a homogeneous mixture of plasma, red and white blood cells, and platelets, with these components evenly distributed throughout the liquid.
Alloy steel: Alloy steel is a homogeneous mixture of iron and other metals, such as nickel, chromium, or manganese, with the metals evenly distributed throughout the alloy.
Soft drinks: Soft drinks are homogeneous mixtures of water, sugar, and other additives, with the components evenly distributed throughout the liquid.
Overall, homogeneous mixtures are common in nature and in many industrial processes, and their uniformity allows for consistent properties and behaviors.
To know more about homogeneous mixture click here:
brainly.com/question/24898889
#SPJ4
Carbon dioxide consists of two oxygen atoms bonded on either side of a carbon atom, with separations of about 116.3pm between carbon and oxygen, and about 232.6pm between the two oxygens. Suppose that we model carbon dioxide as two −2e point charges, the oxygens, electrically bonded to a +4e charge in the center, the carbon, as depicted in the diagram below. What is the electric potential energy of this configuration of charges?
Answers
The electric potential energy of this configuration of charges is −71.92 nJ, which when rounded to two decimal places is −150 nJ.
Diagram of carbon dioxide:
O
//
O = C = O
\\
O
In the given diagram, the two oxygen atoms have a charge of −2e, and the carbon atom has a charge of +4e.
The electric potential energy of this configuration of charges is given by the equation:
Electric potential energy = (kq1q2) / d
where k is Coulomb's constant, q1 and q2 are the charges of the two point charges, and d is the distance between them.
The value of k is 8.99 × 109 Nm2/C2, q1 and q2 are −2e and +4e respectively, and the distance between them is 116.3 pm + 232.6 pm = 348.9 pm = 3.489 × 10⁻⁹ m.
The electric potential energy of this configuration of charges is:
Electric potential energy = (kq1q2) / d= (8.99 × 10⁹ × (-2e) × 4e) / (3.489 × 10⁻⁹)
= -71.92 × 10⁻⁹ J
= -71.92 nJ (rounded to two decimal places)
Therefore, the electric potential energy of this configuration of charges is −71.92 nJ, which when rounded to two decimal places is −150 nJ.
Learn more about carbon dioxide from this link:
https://brainly.com/question/28157529
#SPJ11
T/F: the step in any reaction sequence determines the rate law for the overall reaction. this step is called the rate- step.
Answers
The step in any reaction sequence that determines the rate law for the overall reaction is called the rate-determining step. TRUE.
This step is also known as the slowest step in the reaction sequence. The rate law for the overall reaction is determined by the reactants and the rate-determining step. Therefore, it is important to identify the rate-determining step in order to determine the rate law for the overall reaction.
True, the step in any reaction sequence that determines the rate law for the overall reaction is called the rate-determining step. This step has the slowest rate among all the steps in the reaction sequence and thus governs the overall rate of the reaction.
Learn more about reaction sequence
brainly.com/question/29607707
#SPJ11
A bulb is found to weigh 5.76 grams more when filled with air at 298 K and 1.00 atmosphere pressure than when it is evacuated. What is the volume of the bulb? (Consider air to be 80% nitrogen and 20% oxygen by volume)
Answers
Answer:
Subtract water vapor pressure from total pressure to get partial pressure of gas A: PA=1.03 atm- 1 atm=0.03 atm.
What is the total pressure of the gases at 298 K?
98.8 kPa
A sample of nitrogen gas is bubbled through water at 298 K and the volume collected is 250 mL. The total pressure of the gas, which is saturated with water vapour, is found to be 98.8 kPa at 298 K.
The total pressure of a mixture of gases can be defined as the sum of the pressures of each individual gas: Ptotal=P1+P2+… +Pn. + P n . The partial pressure of an individual gas is equal to the total pressure multiplied by the mole fraction of that gas.
How do you find the partial pressure of water in air?
e is vapor pressure Rv = R∗/Mv = 461.5Jkg−1K−1 and Mv = 18.01gmol−1, ϵ = Mv/Md = 0.622. The vapor pressure is the partial pressure of the water vapor. where es is in Pascals and T is in Celsius.
ExpHow do you find the pressure of h2?
For the high pressures in which hydrogen gas is often stored, the van der Waals equation can be used. It is P+a(n/V)^2=nRT. For diatomic hydrogen gas, a=0.244atm L^2/mol^2 and b=0.0266L/mol.lanation:
Se hacen reaccionar 100 g del agente oxidante con 25 g del agente reductor, según la reacción REDOX LaTeX: N_2\left(g\right)+H_2\left(g\right)\longrightarrow NH_3\left(g\right)N 2 ( g ) + H 2 ( g ) ⟶ N H 3 ( g ) Indique el reactivo en exceso y los gramos de amoniaco formado, si la eficiencia del proceso es del 80 %
Answers
Answer:
El reactivo en exceso es hidrógeno
97.12g NH₃ son formados
Explanation:
Basados en la reacción:
N₂(g) + 3 H₂(g) → 2 NH₃(g)
El hidrógeno pasa de estado de oxidación 0 a estado de oxidación +1. Al perder un electrón se oxida y es el agente reductor.
El nitrógeno pasa de estado 0 estado -3. Al ganar 3 electrones se reduce y es el agente oxidante.
100g de N₂ son (Peso molecular: 28g/mol):
100g × (1mol / 28g) = 3.57 moles de N₂
Y 25g de H₂ son (Peso molecular: 2g/mol):
25g × (1mol / 2g) = 12.5 moles de H₂
Como 3 moles de hidrógeno reaccionan por mol de nitrógeno, las moles de nitrógeno que se necesitan para hacer reaccionar completamente 12.5 moles de hidrógeno son:
12.5 moles H₂× (1 mol N₂ / 3 moles H₂) = 4.17 moles de nitrógeno.
Como hay 3.57 moles de nitrógeno, el reactivo en exceso es hidrógeno.
Como el reactivo limitante es el nitrógeno y 1 mol de nitrógeno produce 2 moles de amoniaco, las moles de amoniaco son:
3.57 moles de N₂ × (2 moles NH₃ / 1 mol N₂) = 7.14 moles de NH₃
La masa producida idealmente es:
7.14 mol NH₃ ₓ (17g/mol) = 121.4 g de NH₃. Como la eficiencia del proceso es del 80%:
121.4 g NH₃ × 80% = 97.12g NH₃ son formados
Answer:
Excess reactant: H₂
Mass of produced ammonia, 97.1 g
Explanation:
Identify the reaction:
N₂ + 3H₂ → 2NH₃
We identify the reducing agent and the oxidizing agent:
N₂ changed the oxidation state from 0 to -3. This is the reduction, so it is the oxidizing agent. By the way the H₂ is the reducing agent.
We convert the mass to moles:
100 g / 28 g/mol = 3.57 moles of N₂
25 g / 2 g/mol = 12.5 moles of H₂
Ratio is 1:3. For 1 mol of nitrogen, we need 3 moles of hydrogen
Then, 3.57 moles of N₂ would need (3.57 . 3) / 1 = 10.7 moles
We have 12.5 moles of H₂, so the hydrogen is the excess reactant and the nitrogen is the limiting.
To produce ammonia, the reaction needs 1 mol of N₂, that can produce 2 moles of product
3.57 moles of N₂ will produce (3.57 . 2) / 1 = 7.14 moles of NH₃
As yield reaction is 80%, we will produce 7.14 mol . 0.80 = 5.71 moles
We convert the moles to mass: 5.71 mol . 17 g / 1mol = 97.1 g
How many protons does one oxygen atom contain
Answers
Answer:
8
Explanation:
Its official chemical symbol is O, and its atomic number is 8, which means that an oxygen atom has eight protons in its nucleus
I need help on this question

Answers
at the end of glycolysis, in what molecule(s) can one find the energy that was contained in the chemical bonds of glucose? select all that apply.
Answers
At the end of glycolysis, one can find the energy that was contained in the chemical bonds of glucose in ATP, NADH and pyruvate molecule.
The process of glycolysis takes place in the cytosol of a cell. It can be broken down into two phases viz. energy requiring phase and energy releasing phase.
Energy requiring phase: The starting molecule i.e glucose is at first rearranged and two phosphate groups are attatched to it, which comes from ATP. The phosphate groups form the sugar- fructose-1,6-biphosphate. This splits into three-carbon sugars
Energy releasing phase: The three carbon-sugars are converted into three-carbon molecules i.e two ATP molecules and one NADH molecule and pyruvate.
Thus, in ATP, NADH and pyruvate we can find the energy contained in chemical bonds of glucose.
To know more about glycolysis here
https://brainly.com/question/15250847
#SPJ4
a compound was found to contain 68.42% Cr and the rest oxygen by mass. what is the empirical and molecular formula of the substance? the compound has a molecular weight of 456.00 g/mol
Answers
Given that the compound contains 68.42% chromium and the rest oxygen, we can assume that the total mass of the compound is 100 grams. Therefore, the mass of chromium in the compound is:mass of Cr = 68.42 The mass of oxygen in the compound is:mass of O = 100 g - 68.42 31.58 g.
What is a compound ?A compound is a substance that is made up of two or more different elements that are chemically bonded together in a fixed ratio. Compounds can be formed through chemical reactions between atoms, ions, or molecules, and they can have a wide range of physical and chemical properties.
Compounds are distinct from mixtures, which are combinations of substances that are not chemically bonded together and can be separated by physical means. In contrast, the atoms in a compound are held together by strong chemical bonds, and the compound has a unique set of properties that are different from those of its constituent elements.
Examples of common compounds include water (H2O), carbon dioxide (CO2), sodium chloride (NaCl), and glucose (C6H12O6). Compounds play an important role in many areas of science and industry, including medicine, agriculture, materials science, and environmental science.
To know more about Compounds visit :
https://brainly.com/question/30904369
#SPJ1
A solid conducting sphere of radius R has a potential at the surface of V with respect to that creates an electric field E. What is the electric field at a radius of R/2? a. E b. 2E c. Impossible to know d. [infinity]
e. E/2
f. 0
Answers
The electric field at a radius of R/2 is impossible to know from the information given. The correct answer is Option C.
The potential difference is proportional to the electric field, but the relationship between the potential difference and the electric field inside a conductor depends on the distribution of charges within the conductor.
Since the sphere is solid and the information about the distribution of charges within the sphere is not provided, it is impossible to determine the electric field at a radius of R/2.
Additionally, the electric field inside a conductor is always zero, so the electric field at R/2 cannot be equal to 2E or E/2.
To read more about electric field, Visit- https://brainly.com/question/8971780
#SPJ11
what is the binding energy (in kj/mol) for ag-107? the mass of a hydrogen atom is 1.00783 amu, the mass of a neutron is 1.00867 amu, and the atomic mass of this isotope is 106.90509 amu.
Answers
The binding energy of Ag-107 is 2.86 x 10¹² J/mol.
What is the binding energy for Ag-107?The binding energy of Ag-107is determined as follows:
Mass of 61 protons and 46 neutrons = 61(1.00783 amu) + 46(1.00867 amu) = 107.90562 amu
Actual mass of Ag-107 = 106.90509 amu
Mass defect = (107.90562 amu - 106.90509 amu)
Mass defect = 0.00053 amu
The binding energy can be calculated using the equation:
E = Δmc₂
where;
Δm is the mass defect,c is the speed of light, andE is the binding energy.Solving for E:
E = (0.00053)(1.66054 x 10⁻²⁷)(2.998 x 10⁸)²
E = 4.75 x 10⁻¹¹ J
Convert this value to kilojoules per mole:
E = (4.75 x 10⁻¹¹)(6.022 x 10²³) / 1000
E = 2.86 x 10^12 J/mol
Learn more about binding energy at: https://brainly.com/question/23020604
#SPJ1
suppose there is a gaseous mixture of nitrogen and oxygen. if the total pressure of the mixture is 490 mmhg , and the partial pressure of nitrogen is 210 mmhg , calculate the partial pressure of oxygen in the mixture using dalton's law.
Answers
The partial pressure of Oxygen in the mixture is 280 mmHg.
The Sum of the partial pressure of elements in a solution equals the total pressure of the mixture as stated by Dalton's Law.
Total pressure = Partial pressure of Nitrogen + Partial pressure of Oxygen
P' = P₁ + P₂
The partial pressure is the pressure that each gas would exert if it alone occupied the volume of the mixture at the same temperature.
The system responds to changes in pressure, temperature, and chemical composition, phases may be created, eliminated, or altered in composition.
∴ 490 = 210 + P ₂
P₂ = 280 mm of Hg
Hence, the partial pressure of oxygen gas is 280 mm Hg.
Learn more about Dalton's law in
brainly.com/question/14119417
#SPJ4
1.) what is the name of NaF formula
Answers
Answer:
\(\text{Sodium Fluoride}\)
Explanation:
Here, we want to get the name for the given chemical formula
Looking at the formula, we look at the names of the element
The names are sodium and fluorine
We write the name of the metal element first, then the non-metal atom
Thus, we have it as
\(\text{Sodium Fluoride}\)At th williams field the summers are short
Answers
Williams Field experiences short, chilly summers, lengthy, chilly, snowy, windy winters, with seasonal partial cloudiness. The average annual temperature ranges from -20°F to 32°F, with uncommon excursions below -32°F or as high as 37°F.
Climatic Conditions at the Williams Field
From November 23 to February 7 is the warm season, which lasts 2.5 months and has an average daily high temperature of more than 24°F. Williams Field experiences its warmest weather in January, with an average high temperature of 31°F and low temperature of 23°F.
The average daily high temperature during the 5.3-month cold season, which runs from April 19 to September 28, is below -2°F. With an average low of -20°F and a high of -10°F, August is the coldest month of the year at Williams Field.
To learn more about Climatic Conditions
https://brainly.com/question/29332636
#SPJ1
The actual size of a wood louse is 0.4mm but Robert drew his 5mm. What is the magnification?
Please answer QUICK I ain’t got much time
I’ll give brainiest if you answer in the next 15 mins
Answers
Answer:
Magnification is the number of times larger an image is compared with the real size of the object.
Magnification = Image / Actual = 5/0.4 =12.5
Explanation:
How many grams of potassium iodide will dissolve in 500 grams of water at 20 degrees C?
Answers
Answer:
Solubility range of KI in 500 grams of water at 20 degrees C is 700-740gm
Explanation:
Using the solubility curve of the solubility of KI with the given temperature
we infer from the graph that at 20 degrees Celsius the solubility of KI is 140-148 grams per 100 grams of water.
so for 500 grams of water the solubility of KI will be
= 140*5= 700gm to 148*5= 740
Solubility range of KI in 500 grams of water at 20 degrees C is 700-740gm
why do na+ ions enter the cell when voltage-gated na+ channels are opened in neurons?
Answers
When voltage-gated Na+ channels are opened in neurons, Na+ ions enter the cell due to the principles of electrochemical gradient and membrane potential.
The opening of voltage-gated Na+ channels is triggered by a depolarization of the cell membrane, often caused by an action potential. When the membrane depolarizes, the voltage-gated Na+ channels undergo a conformational change, allowing Na+ ions to flow into the cell.
The movement of Na+ ions is driven by two factors:
1. Electrochemical gradient: Na+ ions have a higher concentration outside the cell compared to inside. This concentration gradient tends to drive Na+ ions into the cell.
2. Membrane potential: The inside of the cell is negatively charged relative to the outside due to an uneven distribution of ions. This creates an electrical potential across the cell membrane. When the voltage-gated Na+ channels open, the membrane potential becomes more positive, attracting Na+ ions into the cell.
As a result, the combination of the concentration gradient and the membrane potential leads to the influx of Na+ ions into the cell when the voltage-gated Na+ channels are opened in neurons.
This influx of Na+ ions is critical for the generation and propagation of action potentials, which are essential for neuronal communication and the functioning of the nervous system.
To learn more about voltage-gated refer here:
https://brainly.com/question/28588960#
#SPJ11
In the bicarbonate buffering system, carbon dioxide and water join to form ___________, which dissociates into hydrogen and ________________.
Answers
In the bicarbonate buffering system, carbon dioxide and water join to form carbonic acid, which dissociates into hydrogen and bicarbonate.
Why bicarbonate is an excellent buffering system?Bicarbonate is an excellent buffering system because this compound can bind free hydrogen ions (H+) and thus the system avoids sudden changes in the pH of a solution.
In conclusion, in the bicarbonate buffering system, carbon dioxide and water join to form carbonic acid, which dissociates into hydrogen and bicarbonate.
Learn more about the buffering systems here:
https://brainly.com/question/22821585
#SPJ1
a reversible reaction involving chemicals in a variety of physical states can reach a(n) equilibrium. endothermic
Answers
It is true that a reversible reaction involving chemicals in a variety of physical states can reach equilibrium, whether it is endothermic or exothermic.
At equilibrium, the rates of the forward and reverse reactions are equal, and the concentrations of reactants and products remain constant over time. The equilibrium constant (Kc) is a measure of the extent to which a reaction proceeds towards products or reactants at equilibrium, and it depends on the temperature and pressure conditions of the system. It is true that a reversible reaction involving chemicals in a variety of physical states can reach equilibrium, whether it is endothermic or exothermic.Exothermic is a term used to describe a chemical reaction that releases heat energy to the surroundings. In an exothermic reaction, the products formed have less energy than the reactants, and the difference in energy is released as heat. This heat can be felt as an increase in temperature or as the emission of light or sound.Examples of exothermic reactions include combustion reactions, such as the burning of fuels like wood or gasoline, which release heat and light. Another example is the reaction between sodium hydroxide and hydrochloric acid, which releases heat and produces water and salt. Exothermic reactions are often used in applications such as energy production and heating.
To know more about exothermic visit :
https://brainly.com/question/31214950
#SPJ11
The body's reaction to a change in the environment is called a response.
Please select the best answer from the choices provided
OT
OF

Answers
Explain why or why you would expect bisulfate to be a good leaving group for substitution reaction?
Answers
Due to the presence of sulfonic acid functional group, bisulfate is considered a good leaving group for substitution reaction.
A substitution reaction is a chemical reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. A leaving group is a part of a molecule that takes with it a pair of electrons when it departs from the molecule. It is a species that can accept a pair of electrons to form a new bond.
A good leaving group is generally an anion that is either neutral or a weak base.
In organic chemistry, bisulfate is a good leaving group for substitution reactions because it is an excellent leaving group due to its sulfonic acid functional group, which makes it a strong acid. The negatively charged oxygen atom can stabilize the negative charge created when it departs from the molecule by donating its lone pair of electrons. As a result, the sulfonic acid's anionic character, which makes it a good leaving group.
Because the molecule's ability to donate its lone pair of electrons stabilizes the leaving group, a compound with a better leaving group will be able to perform substitution more readily. This makes bisulfate an excellent leaving group for substitution reactions.
Thus, the reason is sulfonic acid functional group.
To learn more about susbtitution reaction :
https://brainly.com/question/10143438
#SPJ11
4. They are the muscles which have complete control and responsible for all
kinds of body movement
A. Contracting B. Involuntary C. Relaxing D. Voluntary
5. What system that is made up of many bones joined to form a structure?
A. Digestive B. Integumentary C. Muscular
D. Skeletal
6. How do our muscles work?
D. By touching
A. By pulling B. By smelling C. By seeing
7. These are the long bones of our body.
A legs B. Patella
C. Ribs D. Tendons
8. What composes the lower extremities of the body?
A backbone B. Compact bone C. Knee bone D. Pelvic bone
9. An organ that manufactures the blood cells in our body?
A Bone marrow B. Compact bone C. Knee bone D. Pelvic bone
10. Are muscles that move without conscious effort like the beating of our heart.
A contracting B. Involuntary
C. Relaxing
D. Voluntary
Answers
Option B: voluntary muscles are the muscles which have complete control and are responsible for all kinds of movements.
Animals' muscles are the types of tissue that cause movement or motion. Voluntary refers to action taken of one's own free will or will. All of the muscles that are linked to the skeleton are voluntary muscles, hence these muscles are also referred to as skeletal muscles or striated muscles because of the way their muscle fibres give them a striated, or stripy, appearance. This, therefore, tells us that option B is he right choice.
The only muscles that can be actively manipulated are those in the skeleton. Since the muscles are connected to the bones, pulling on them moves the bones. Skeletal muscles are used for every conscious movement a person makes.
To know more about skeletal muscles, refer to the following link:
https://brainly.com/question/12252128
#SPJ4
Calculate the molar mass of 1 mol Br2 = g

Answers
1) Convert moles of Br2 to grams
The molar mass of Br2 is 159.81 g/mol.
\(gBr_2=1molBr_2\cdot\frac{159.81gBr_2}{1molBr_2}=159.81gBr_2\)1 mol of Br2 is equal to 159.81 g Br2.
A number that tells how many molecules there is of a certain compound is called a(n)_
*
20 points
subscript.
reaction.
coefficient.
formula.
Answers
A number that tells how many molecules there are of a certain compound is called a(n) coefficient.
A subscript is a small-sized variety on the bottom right of the image. It refers to the number of atoms of the detail. If the subscript seems on the lowest left of the image, it offers the detail's atomic range.
The chemical formulation which represents the actual range of atoms of every detail present in a molecule is called molecular formula.
The coefficients suggest the range of each substance worried inside the response and may be changed so that it will stabilize the equation. The coefficients deliver the number of molecules worried inside the response.
In the example reaction, molecules of hydrogen react with one molecule of oxygen and convey molecules of water. second: the coefficients deliver the range of moles of every substance concerned within the reaction.
Learn more about coefficient here:-https://brainly.com/question/1038771
#SPJ1