Answers
Answer:
Hope this helped :) good luck! ❤️
Explanation:
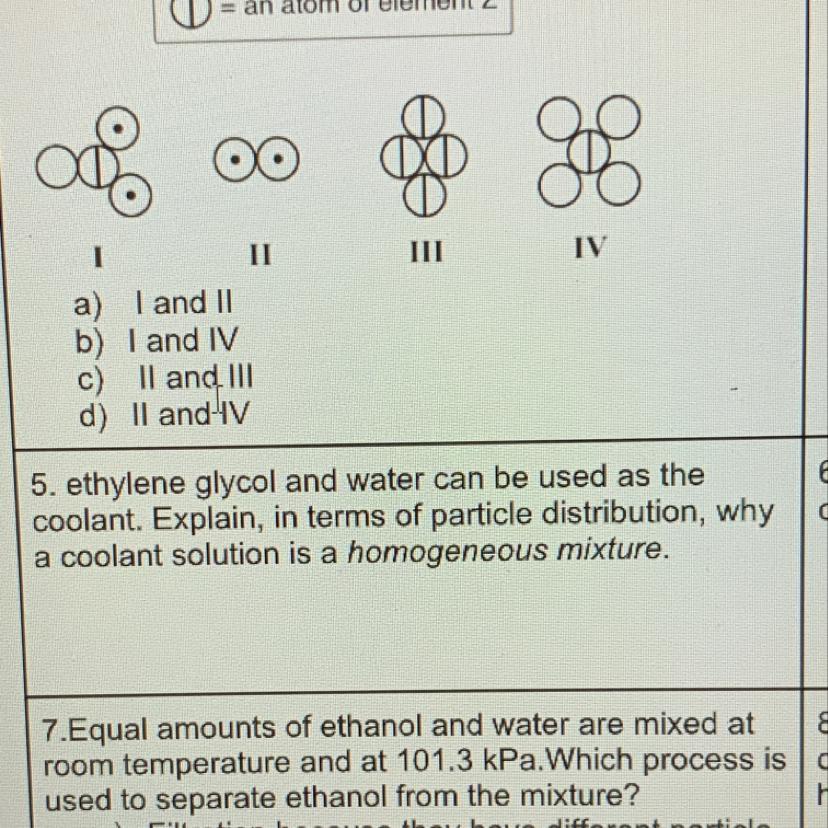
A coolant solution is a homogeneous mixture because the coolant particles are not chemically combined with the water (keep their properties) and they are evenly distributed throughout the water.
Related Questions
The rate constant for the reaction below was determined to be 3.241×10-5 s–1 at 800 K. The activation energy of the reaction is 255 kJ/mol. What would be the value of the rate constant at 9.50×102 K?
Answers
Answer:
\(k_2=1.379x10^{-2}s^{-1}\)
Explanation:
Hello.
In this case, for a normal reaction, when we know the rate constant at a determined temperature, we can compute at another temperature via the Arrhenius equation specified for temperature change:
\(k_2=k_1exp[-\frac{Ea}{R}(\frac{1}{T_2}-\frac{1}{T_1} )]\)
In such a way, the rate constant at 9.50x10² K is:
\(k_2=3.241x10^{-5}s^{-1}exp[-\frac{255000}{8.314}(\frac{1}{950}-\frac{1}{800} )]\\\\k_2=1.379x10^{-2}s^{-1}\)
Best regards!
A person has 5.0 liters of blood which contains 95 mg of glucose per 100 ml. What is the total mass of glucose (in grams) in the 5.0 liters of blood
Answers
Don’t forget the significant figures, 4.75 is rounded to 4.7 because the significant figures in this case is 2 sig figs (5.0 liters of blood).
A compound is 60.00% carbon, 5.75% hydrogen, and 34.25% oxygen. Determine the empirical formula of the compound.
Answers
Answer:
The empirical formula of the compound is C2H5O.
Calculate your total body volume in liters assuming an average body density of 0.90 g/ml. (Use 130 lbs.
as your body weight.)
Answers
The volume of a substance is its mass divided by density. If the mass of your body is 130 lbs or 58.9 kg, then, volume is 65 L.
What is density ?Density of substance is the measure of its mass per unit volume. It describes how closely its particles are packed. Density depends on the bond type, temperature and pressure.
Volume of the object is the space occupied by its particles. Volume can be expressed in L, ml, cm³, dm³ etc. Only solids and liquids has a definite volume and the volume of gases is that of the container.
Give that, mass of body = 130 lbs
density = 0.90 g/ml
1 lbs = 0.453 kg or 453.5 g
then , 130 lbs = 58900 g or 58.8 kg
volume = mass/density
= 58900g / 0.90 g/ml
= 65444ml =65.44 L.
Therefore, the volume of our body will be 65.44 L.
Find more on density:
brainly.com/question/15164682
#SPJ1
How many milliliters of gasoline have a mass of 2.4 kg ( D=0.74g/mL )?
Answers

Answer:
3243.2ml
Explanation:
Converting 2.4kg to g = 2.4X1000
=2400g
Since Density is mass/volume,
then volume is = mass/density
volume = 2400/0.74
=3243.2ml
NaOH is the limiting reactant, producing
2.0 mol Na3PO4. What mass of
Na3PO4 forms during the reaction?
Na3PO4: 164 g/mol
[?] g Na3PO4
Report your answer to two significant figures.
g Na PO
4
Enter
Answers
The mass of Na₃PO₄ formed during the reaction is 328 g.
The balanced chemical equation for the reaction is:
\(3NaOH + Na_3PO_4 - > 3Na_2PO_4 + H_2O\)
From the equation, we can see that 3 moles of NaOH produce 1 mole of Na₃PO₄.
Given that 2.0 moles of Na₃PO₄ is produced, we can set up a proportion to find the amount of NaOH required:
3 mol NaOH / 1 mol Na₃PO₄ = x mol NaOH / 2.0 mol Na3PO4
Solving for x, we get:
x = (3 mol NaOH / 1 mol Na₃PO₄) × (2.0 mol Na₃PO₄ / 1) = 6.0 mol NaOH
So, 6.0 moles of NaOH are required to produce 2.0 moles of Na₃PO₄.
To find the mass of Na₃PO₄ produced, we can use its molar mass:
mass = moles × molar mass = 2.0 mol × 164 g/mol = 328 g
Therefore, the mass of Na₃PO₄ formed during the reaction is 328 g.
learn more about limiting reactants here
https://brainly.com/question/26905271
#SPJ1
? C+? O2 +? CO2,
what is the maximum amount of CO2 which
could be formed from 13.19 g of C and 15.92 g
of O2?
Answer in units of g.
Answers
Look at the mole ratio in the balanced equation
2CO + O2 ==> 2CO2
3.44 mol O2 x 2 mol CO/mol O2 = 6.88 moles CO
convert 6.8 × 10^2 to standard notation.
Answers
Answer:
680
Explanation:
Move the decimal 2 to the right.
When a number is written using only the digits of the number, that is considered standard notation. 6.8 × 10² is converted into standard notation by shifting two decimal to the right side, then it became 680.
What is standard notation ?A form of writing a certain number, an equation, or an expression in a way that adheres to set norms is known as a standard notation. For instance, 4,500,000,000 years is how 4.5 billion years is written.
When a number is written using only the digits of the number, that is considered standard notation. Since words are not used in conventional number notation, this is the way that numbers are typically written.
The stages to writing a number in its standard form are as follows: Write the first digit of the supplied number in step one. Step 2: After the first number, add the decimal point. Step 3: Next, count how many digits there are in the supplied number after the first one and express that number as a power of 10.
Thus, the standard notation is 680.
To learn more about standard notation, follow the link;
https://brainly.com/question/10253124
#SPJ2
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
A compound which is an electron pair donor is generally classified as a????? urgent please
Answers
Answer:
A Lewis base is defined as any species that can donate a pair of electrons, and a Lewis acid is any species that can accept a pair of electrons. All Brønsted–Lowry bases (proton acceptors), such as OH−, H2O, and NH3, are also electron-pair donors.
What is the role of a decomposer in a food chain?
A. to move food from producers to consumers
B. to move food from consumers to other consumers
C. to make food for the ecosystem
D. to return matter to the environment
Answers
Answer:D
Explanation:
Decomposers decompose food and return it to the environment through the soil
Consider the structure of arginine in its fully protonated state with charge +2.
Give the pa value for the α‑amino group (group A) of arginine. An answer within ±0.5 units is acceptable.
pa (−NH2)=
Give the pa value for the α‑carboxyl group (group B) of arginine. An answer within ±0.5 units is acceptable.
pa (−COOH)=
Give the pa value for the guanidino side chain group (group C) of arginine. An answer within ±0.5 units is acceptable.
pa (−NH−(NH2)C=NH)=
Calculate the pI of arginine. Give your answer to two decimal places.
pI=

Answers
For arginine, the pKa value for group A is approximately 9.5.For arginine, the pKa value for group B is approximately 2.2. In arginine, the pKa value for group C is approximately 12.5.the pI of arginine is approximately 11.00.
The pKa values for the different ionizable groups in arginine are as follows:
Group A: α-amino group (-NH2)
Group B: α-carboxyl group (-COOH)
Group C: guanidino side chain group (-NH-(NH2)C=NH)
For group A, the pKa value represents the pH at which half of the amino groups are protonated and half are deprotonated. The pKa of an α-amino group is typically around 9.5 to 10.5. For arginine, the pKa value for group A is approximately 9.5.
For group B, the pKa value represents the pH at which half of the carboxyl groups are protonated and half are deprotonated. The pKa of an α-carboxyl group is typically around 2.0 to 2.5. For arginine, the pKa value for group B is approximately 2.2.
For group C, the guanidino side chain contains multiple ionizable sites. The pKa value for this group is more complex and can vary depending on the specific environment and neighboring groups. In arginine, the pKa value for group C is approximately 12.5.
To calculate the pI (isoelectric point) of arginine, we need to find the pH at which the net charge of the molecule is zero. This occurs when the positive and negative charges on the molecule balance each other out. Since arginine has a net charge of +2 in its fully protonated state, the pI can be calculated as the average of the pKa values for the two groups that contribute to the positive charge.
pI = (pKa of group A + pKa of group C) / 2
pI = (9.5 + 12.5) / 2
pI = 11.0
For more such questions on arginine visit:
https://brainly.com/question/30483159
#SPJ8
On Earth he weighs 720 newtons. List of weights: 655 N; 1,872 N; 792 N; 36 N; and 661 N. What planets does he visit?
Answers
On Earth he weighs 720 newtons. 36N is the weight. Therefore, the correct option is option D among all the given options.
What is weight?The gravitational force of attraction exerted on an item by the presence of a huge second object, including the Earth or Moon. Weight is a result of the fundamental law of gravitation: whatever two things have the same weight.
They attract each other using a force that really is directly related to the sum of their masses as well as inversely related to the square of something like the distance separating them due to their masses.
F = mass ×4/9g
= 720 ×4/9
=36N
Therefore, the correct option is option D.
To know more about weight, here:
https://brainly.com/question/12156778
#SPJ9
Perform the following operation
and express the answer in
scientific notation.
3.8x10-5 :
8.1x103

Answers
Answer:
\( 4.69135802 \times {10}^{ - 9} \)
Explanation:
\(3.8 \times {10}^{ - 5} \div 8.1 \times {10}^{3} \\ \\ = \frac{3.8 \times {10}^{ - 5} }{8.1 \times {10}^{3}} \\ \\ = 0.469135802 \times {10}^{ - 5} \times {10}^{ - 3} \\ \\ = 0.469135802 \times {10}^{ - 8} \\ \\ = 4.69135802 \times {10}^{ - 9} \)
Answer:
4.69135802x10^-9
Explanation:
This is easy
Rubbing alcohol evaporates from your arm quickly, leaving a cooling effect on your skin. How do the molecules of gas compare to the molecules as a liquid?
The gas particles move faster, have the same molecular composition, and have weaker attractions between them than the liquid particles.
The gas particles move faster than the liquid particles, and the bonds of the molecules are broken during evaporation to allow gas atoms to spread apart.
The gas particles move slower but have the same molecular structure and the same attraction between them as the liquid particles.
The gas and liquid particles move at the same speed, but the bonds of the molecules are broken during evaporation to allow the gas atoms to spread apart.
Answers
The gas particles move faster than the liquid particles, and the bonds of the molecules are broken during evaporation to allow gas atoms to spread apart.
EvaporationWhen substances evaporate from liquid states to gaseous states, the bonds between the molecules of the substances are broken and the atoms spread apart as far away as possible.
In addition to the breaking of bonds, the particles of the gases move faster than when they were linked together in liquids since the particles are now free from one another.
More on evaporation can be found here: https://brainly.com/question/5019199
#SPJ1
other m Ammonium nitrate decomposes to nitrogen(1) oxide and water. 9. Some oxides of nitrogen are atmospheric pollutants. and oxygen. Revision Exercise When compound X is heated, a red-brown gas is evolved and a yellow residue is left on cooling. Name: (i) The red-brown gas. (ii) The ions present in the residue. (ui) Compound X.
Answers
We can determine the following based on the provided information:
Metal nitrate A is a compound that, when heated, transforms into colourless gas, brown gas B, and a metal oxide with a yellowish brown hue. B. C: Colourless petrol C. B: Brown petrol C. D: Compound D, a yellow precipitate produced by the reaction of potassium iodide with an aqueous solution of compound A.
We may deduce that A is probably lead nitrate (Pb(NO3)2) because lead is frequently used in soldering alloys and the metal contained in A is utilised in an alloy for soldering purposes.
Identifications:
Lead nitrate, or Pb(NO3)2,
N2O: Nitrogen dioxide
B: Carbon (CO)
D: PbI2, or lead iodide.
Thus, this can be concluded regarding the given scenario.
For more details regarding metals, visit:
https://brainly.com/question/29404080
#SPJ1
Your question seems incomplete, the probable complete question is:
A metal nitrate A on heating gives a yellowish brown coloured metal oxide along with brown gas B and a colourless gas C. An aqueous solution of A on reaction with potassium iodide forms a yellow precipitate of compound D. Identify A, B, C and D. Also, identify the types of reactions taking place. Metal present in A is used in an alloy which is used for soldering purposes.
Chlorine gas reacts with fluorine gas to form chlorine trifluoride. Cl2(g)+3F2(g)→2ClF3(g) A 2.05 L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmHg and fluorine gas at a partial pressure of 730 mmHg .
Answers
Answer:
2.4 grams of ClF3
Explanation:
First let us determine the moles of Cl2 and F2,
Cl2 = ( ( 337 )( 2.05 L ) / ( 0.082 )( 298 K ) ) * ( 1 atm / 780 ),
Cl2 = ( 690 / 24.436 ) * ( 1 / 780 ),
Cl2 = ( About ) 0.036 moles of Cl2
_________________________________________________
F2 = ( ( 729 )( 2 L ) / ( 0.082 )( 298 K ) ) * ( 1 atm / 780 ),
F2 = ( 1458 / 24.436 ) * ( 1 / 780 )
F2 = ( About ) 0.078 moles of F2
Now let us identify the limiting reactant, considering the ratio between ClF3 and Cl2 / F2. In this case F2 is the limiting reactant, as it forms a smaller molar ratio;
The theoretic yield is thus performed with the limiting reactant F2,
0.078 * ( 2 / 3 ) * ( 92.45 / 2 ) = ( About ) 2.4 grams of ClF3
The average kinetic energy of the particles in a sample of
matter at 600 K is
as the average kinetic energy of the
particles in another sample at -73°C.
Answers
This means that the average kinetic energy of the particles in the first sample (at 600 K) is three times greater than the average kinetic energy of the particles in the second sample (at -73°C or 200 K).
According to the kinetic theory of gases, the average kinetic energy of gas particles is directly proportional to the temperature of the gas. The equation that relates the average kinetic energy (KE) and temperature (T) of a gas is
KE ∝ T
the average kinetic energy of gas particles increases with an increase in temperature and decreases with a decrease in temperature.
Given that one sample is at 600 K and the other sample is at -73°C, one need to convert -73°C to Kelvin by adding 273 (Kelvin = Celsius + 273).
The temperature in Kelvin for the second sample is: T₂ = -73°C + 273 = 200 K
Now one can compare the average kinetic energies of the two samples.
KE₁ / KE₂ = T₁ / T₂
Substituting the given temperatures: KE₁ / KE₂ = 600 K / 200 K
Simplifying the equation: KE₁ / KE₂ = 3, the average kinetic energy of the particles in the first sample (at 600 K) is three times greater than the average kinetic energy of the particles in the second sample (at -73°C or 200 K).
Learn more about the kinetic energy here
https://brainly.com/question/999862
#SPJ1
how polar and nonpolar substances interact with one another. Why do they or don’t they mix?
Answers
Answer:
interact with each other through London dispersion forces.
Electrochemical cells generate electricity from which of the following? Select all that apply.
electron transfer
flow of electrons
dissolving an ionic compound
redox reactions
Answers
By a redox reaction that involves the transfer of electrons, often through the dissolution of an ionic substance, electrochemical cells produce electricity from the flow of electrons.
What fuels the production of energy by electrochemical cells?In electrochemistry, redox or oxidation-reduction reactions, in which electrons travel from one element to another, can produce electricity. Redox processes involve the transfer of electrons from one substance to another.
In what element are electrochemical cells made?Batteries use a very significant class of oxidation and reduction reactions to produce useable electrical energy. Using solutions of respective sulphates, copper and zinc metals can be combined to create a straightforward electrochemical cell.
To know more about electrons visit:-
https://brainly.com/question/20513633
#SPJ1
How many grams of the electrolyte Al2(SO4)3 (molar mass=142 g/mole) are required to make 325 mL of solution having an osmotic pressure of 675 mm Hg at 25 oC?
Answers
Answer: 0.36g
Explanation:
π = iMRT
π = (inRT)/V
π = (imRT)/(MrV)
When we make m the subject of the formula we have;
m = (πMrV)/(iRT)
m=(0.8882atm x 142g/mol x 325x10-3L)/(5 x 0.08206LatmK-1mol-1 x 298.15K)
m = 0.335078176662g = 0.36g
Jean is a crime scene investigator. She needs to draw a sketch that shows the height of the objects in the room. Which type of perspective should she draw?
Answers
One-point perspective is a type of linear perspective where all lines converge to a single vanishing point on the horizon line. This technique is particularly suitable when depicting interior spaces or scenes viewed from a single vantage point.
For Jean to accurately depict the height of objects in the room, she should utilize a one-point perspective drawing. One-point perspective is a type of linear perspective where all lines converge to a single vanishing point on the horizon line. This technique is particularly suitable when depicting interior spaces or scenes viewed from a single vantage point.
By placing the vanishing point on the horizon line, Jean can establish a point of convergence for all vertical lines in the room. The height of objects, such as furniture, walls, or doors, can be represented accurately by drawing vertical lines from their base to the vanishing point. This approach creates a sense of depth and proportion, allowing viewers to perceive the relative heights of objects within the room.
Using one-point perspective will help Jean create a clear and realistic representation of the room's spatial arrangement, enabling her to accurately communicate the height relationships between different objects present in the crime scene.
For more question on perspective
https://brainly.com/question/30088701
#SPJ8
List the number of each type of atom on the left side of the equation
C8H18(l) + 12 O2(g) →8 CO2(g) + 9 H2O(9)
Answers
Answer:
dont do it,it gives u a virus
Explanation:
HNO3 + Sn + H2O → H2SnO3 + NO
By algebraic method pls
Answers
Answer:
3Sn + 4HNO3 + H2O → 3H2SnO3 + 4NO
How many compounds, of the ones listed below, have hydrogen bonding?CH3CH2CH2NH2 CH3CH2NHCH2CH3 (CH3CH2)2NCH2CH32130
Answers
Since CH3CH2CH2NH2 may form intermolecular hydrogen bonds, it has a higher boiling point than other compounds. The hydrogen atoms in (CH3)3N (C H 3) 3 N are joined to the carbon atoms.
Since carbon is not a particularly electronegative atom, it cannot donate hydrogen. There aren't any hydrogen atoms to accept, despite the fact that nitrogen is a strong hydrogen atoms. As a result, CH3CH2CH2CH3 is a nonpolar molecular complex in which induced dipole forces are the only intermolecular forces. When both the donor atom, D, and the acceptor atom, A, are among the highly electronegative elements O, N, or F, hydrogen bonding occurs, as shown by the symbol D-H—-A.
To learn more about hydrogen bonds, click here.
https://brainly.com/question/15099999
#SPJ4
Ethyl chloride, CH3CH2Cl, used to produce tetraethyllead gasoline additive, decomposes when heated, to give ethylene and hydrogen chloride.
CH3CH2Cl(g) → C2H4(g) + HCl(g)
The reaction is first order. In an experiment, the initial concentration was 0.00100 M. After heating at 500ºC for 155 seconds, the concentration of ethyl chloride was reduced to 0.00083 M. What is the concentration of ethyl chloride consumed after a total of 400 seconds?
Answers
The amount of the ethyl chloride is 7.8 * 10^-4 M.
What is the first order reaction?A first order reaction is a type of chemical reaction in which the reaction rate is proportional to the concentration of only one reactant. The rate law for a first order reaction is given by the equation: rate = k[reactant]
Given that;
ln[A] = ln[A]o - kt
[A] = concentration at time t
[A]o = Initial concentration
k = Rate constant
t = time taken
Then;
k =ln[A] - ln [A]o/t
k = ln(0.00083 ) - ln(0.00100)/155
k = -(-7.1 - (-6.9)/155)
k = 6.45 * 10^-4
Then;
ln[A] =ln(0.00100) - (6.45 * 10^-4 * 400)
= -6.9 - 0.258
= -7.158
[A] = e^(-7.158)
= 7.8 * 10^-4 M
Learn more about first order reaction:https://brainly.com/question/12446045
#SPJ1
Pure gold is too soft to be worn as jewelry. What evidence from the passage best supports this conclusion?jewelry, gold is more valuable than silver.
Answers
This is evident from the conclusion because the sentence includes the justification, the appropriate verb, and the correct pronoun, it supports the conclusion that pure gold is too soft.
What is conclusion?Conclusion is defined as the concluding paragraph of a research paper, essay, or article that provides a summary of the whole thing.
It can also be defined as a end part that restates the writing's underlying themes and ideas.
Reiterating the speech's thesis, summarizing the key ideas covered in the speech, and using a finishing device that helps viewers form a lasting impression are the three fundamental components of a good conclusion.
Thus, this is evident from the conclusion because the sentence includes the justification, the appropriate verb, and the correct pronoun, it supports the conclusion that pure gold is too soft.
To learn more about conclusion, refer to the link below:
https://brainly.com/question/28832812
#SPJ1
Please if you could even help with one

Answers
The chart of given following ion is
Protons Electrons Neutrons
1) 6 2 8
2) 6 2 7
3) 8 10 9
4) 8 10 8
What are sub atomic particles ?Simply put, a subatomic particle is a particle that is smaller than an atom. Protons, electrons, and neutrons are the three subatomic particles that may typically be separated from an atom.
A neutral atom has the same number of protons and electrons as protons. The sum of the protons and neutrons in the atom's nucleus determines its mass, which is denoted by the letter M. The number of neutrons is equal to the discrepancy between the atomic number and the mass number of the atom (M) (Z).Learn more about Sub atomic particles here:
https://brainly.com/question/16847839
#SPJ13
A biochemist who is studying the properties of certain sulfur (S)–containing compounds in the body wonders whether trace amounts of another nonmetallic element might have similar behavior. To which element should she turn her attention?
F
As
Se
Cr
P
Answers
By investigating the properties of selenium (Se), the biochemist may gain insights into its potential similarities with sulfur (S) and its role in biological systems
.In the search for a nonmetallic element that might exhibit similar properties to sulfur (S) in the body, the biochemist should turn her attention to selenium (Se). Selenium, like sulfur, belongs to Group 16 (Group VI) of the periodic table, also known as the chalcogens.
Elements in this group tend to have similar chemical behaviors due to the same number of valence electrons.Sulfur is known for its involvement in various biochemical processes, including the formation of disulfide bonds in proteins and as a constituent of important compounds such as cysteine and methionine.
Selenium, being in the same group as sulfur, shares some chemical similarities and can participate in similar biochemical reactions. It is an essential trace element in the body and is a component of certain enzymes and proteins.
For more such questions on biochemist
https://brainly.com/question/28231244
#SPJ8
Calculate the pH when 90.0 mL of 0.200 M HBr is mixed with 30.0 mL of 0.400 M CH₃NH₂ (Kb = 4.4 × 10⁻⁴).
Answers
The pH of the solution is 10.82.
To solve this problem, we need to determine the concentration of the hydronium ion (\(H_3O^+\)) in the solution. This can be done using the following steps:
Write the balanced chemical equation for the reaction between HBr and CH₃NH₂.
HBr + CH₃NH₂ → CH₃NH₃⁺ + Br⁻
Write the expression for the base dissociation constant (Kb) for CH₃NH₂.
Kb = [CH₃NH₃⁺][OH⁻]/[CH₃NH₂]
Calculate the concentration of hydroxide ions (OH⁻) in the solution using the Kb value and the concentration of CH₃NH₂.
Kb = [CH₃NH₃⁺][OH⁻]/[CH₃NH₂]
4.4 × 10⁻⁴ = x² / (0.400 M)
x = 6.63 × 10⁻³ M
[OH⁻] = 6.63 × 10⁻³ M
Calculate the concentration of \(H_3O^+\) using the equilibrium constant for the reaction between HBr and \(H_2O\).
\(HBr + H_2O = H_3O^+ + Br^-\)
\(Kw = [H_3O^+][OH^-] = 1.0 * 10^{-14}\\[H_3O^+] = Kw/[OH-] = 1.51 * 10^{-11}\)
Calculate the pH using the concentration of \(H_3O^+\).
\(pH = -log[H_3O^+]\\pH = -log(1.51 * 10^{-11})\)
pH = 10.82
For more question on pH click on
https://brainly.com/question/172153
#SPJ11