Answers
The mass of I2 reacted is 142.2 g
The mass of PCl3 reacted is 153.4 g
What is the stoichiometry?Stoichiometry is a fundamental concept in chemistry and is used in many different areas of science and industry.
We know that;
Number of moles of the F2 produced = 21.1 g/38 g/mol
= 0.56 moles
If 1 mole of I2 produced 1 mole of F2
Then 0.56 moles of I2 reacted
Mass of the I2 reacted = 0.56 mol * 254 g/mol
= 142.2 g
Number of moles of PCl5 = 234.1 g/208 g/mol
= 1.12 moles
If the reaction is 1:1:1
Mass of the PCl3 reacted = 1.12 moles * 137 g/mol
= 153.4 g
Learn more about stoichiometry:https://brainly.com/question/30215297
#SPJ1
Related Questions
The specific heat capacity of silver is 0.24 J/g °C. How many joules of energy are needed
to warm 0.500 g of silver from 25.0°C to 27.5°C?
Answers
Answer:
0.3 J
Explanation:
The equation for heat capacity is Q = mcΔT where Q is the heat, m is the mass of the substance, c is the specific heat capacity of the substance and delta T is the change in temperature. Plugging those values into the equation, we have Q = (.500)(0.24)(27.5-25) = 0.3
Why do some scientists have difficulty communicating about their research to nonscientists?
Answers
Answer:
Non-scientists aren't exposed to the information that scientists have in their field of study. Scientists also have their research based on facts, while non-scientists can be biased on their beliefs and chose to ignore data and logic.
how many electrons are in 40 Ne?
Answers
40 Ne has 10 electrons which consist of 10 protons and 10 neutrons
Neon is a chemical element which is rare gas or noble gas as it has a stable octet electronic configuration, meaning it has 8 valence electrons in its outermost shell.
Neon belongs to group 0/VIII and period 1 of the periodic table.
What is an element?An element is a substance which takes part in a chemical reaction. There are different classes of elements which includes the metals, nonmetals and the metalloids.
So therefore, 40 Ne has 10 electrons which consist of 10 protons and 10 neutrons.
Learn more about elements:
https://brainly.com/question/1379504
#SPJ1
A student writes down several steps of the scientific method. Put the steps in the best order.
Answers
Answer:
doe sit have a picture-
Explanation:
Which element, when combined with phosphorus, would form an ionic compound?
A) Carbon.
B)Oxygen.
C)Magnesium.
D)Boron.
Answers
Explanation:
An ionic compound is a metal + a nonmetal
Since phosphorus is a nonmetal, you need a metal
Carbon is a nonmetal so it’s not carbon
Oxygen is also a nonmetal so it’s not oxygen
Boron is also a nonmetal so it’s not boron
Magnesium is a metal
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, the correct option is option C.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond.
Since phosphorus is a nonmetal, we need a metal. Out of given option the only element that is metal is Magnesium while others are non metal.
Therefore, the correct option is option C.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ2
Calculate ∆G° for a reaction for which ∆H° = 24. 6 kJ and ∆S° = 132 J/K at 298 K. Is the reaction spontaneous under these conditions?
Answers
The reaction of ∆G° is -14,736 J. A negative ∆G° indicates that the reaction can proceed spontaneously without the input of external energy.
To calculate ∆G° (standard Gibbs free energy change) for a reaction, we can use the equation:
∆G° = ∆H° - T∆S°
Given:
∆H° = 24.6 kJ
∆S° = 132 J/K
T = 298 K
First, we need to convert the units of ∆H° to match the units of ∆S° (kJ to J):
∆H° = 24.6 kJ = 24,600 J
Now, we can substitute the values into the equation to calculate ∆G°:
∆G° = 24,600 J - (298 K) * (132 J/K)
∆G° = 24,600 J - 39,336 J
∆G° = -14,736 J
Since ∆G° is negative (-14,736 J), the reaction is spontaneous under these conditions. A negative ∆G° indicates that the reaction can proceed spontaneously.
Learn more about the Gibbs free energy change here:
https://brainly.com/question/14415025
#SPJ11
Could someone help me answer these questions with the answer and typed steps for how each answer was found? I asked this question previously but, I could not read the handwritten answer.
7. A 25 g soil sample was extracted with 75 mL of NH4OAc (pH 7.0), and the filtrate was analyzed
on an atomic absorption unit. The following results were obtained:
100 mg/L Ca2+, 45 mg/L Mg2+, 85.5 mg/L K+, 94.2 mg/L Al3+ and 8.0 mg/L H+.
a. What is the CEC in cmol(+)/kg for this sample?
b. What is the % B.S. for this soil?
c. What is the % acid saturation for this soil sample?
Answers
The CEC for this soil sample is 675.2 cmol(+)/kg.
The % Base Saturation for this soil sample is approximately 136.62%.
The % Acid Saturation for this soil sample is approximately 60.55%.
To calculate the CEC, % Base Saturation (B.S.), and % Acid Saturation for the given soil sample:
a. Calculation of CEC (Cation Exchange Capacity):
CEC is the sum of exchangeable cations in the soil. From the given results, we have:
CEC = Ca2+ + Mg2+ + K+ + Al3+
CEC = (100 mg/L + 45 mg/L + 85.5 mg/L + 94.2 mg/L) / (25 g / 1000)
CEC = 168.7 mg / (25 g / 1000)
CEC = 675.2 cmol(+)/kg
b. Calculation of % Base Saturation (B.S.):
% B.S. represents the percentage of CEC occupied by base cations. In this case, we consider Ca2+, Mg2+, and K+ as base cations. The formula to calculate % B.S. is:
% B.S. = (Ca2+ + Mg2+ + K+) / CEC * 100
% B.S. = (100 mg/L + 45 mg/L + 85.5 mg/L) / (168.7 cmol(+)/kg) * 100
% B.S. = 230.5 mg / (168.7 cmol(+)/kg) * 100
% B.S. = 136.62%
c. Calculation of % Acid Saturation:
% Acid Saturation represents the percentage of CEC occupied by acid cations, in this case, H+ and Al3+. The formula to calculate % Acid Saturation is:
% Acid Saturation = (H+ + Al3+) / CEC * 100
% Acid Saturation = (8.0 mg/L + 94.2 mg/L) / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 102.2 mg / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 60.55%
Please note that the given values were in milligrams per liter (mg/L), and the CEC and % Saturation values were calculated assuming a conversion from mg/L to cmol(+)/kg using the mass of the soil sample (25 g).
TO know more about CEC (Cation Exchange Capacity)
https://brainly.com/question/30689981
#SPJ11
Predict the products of the following reaction:
RbNO2 +BaCO3->
Answers
Answer:
2RbNO2 + BaCO3 → Rb2CO3 + Ba(NO2)2
Explanation:
The balanced reaction equation is shown below;
2RbNO2 + BaCO3 → Rb2CO3 + Ba(NO2)2
This reaction is possible because the reduction potential of Rb is -2.98V while that of Ba is –2.92 V. Hence Rb can displace Ba from its salt solution.
The equation is balanced since the number of atoms of each element on the left and right hand sides of the reaction equation are equal.
The products for the reaction of Rubidium nitrite and Barium carbonate has been Barium nitrite and Rubidium carbonate.
Rubidium nitrite has been the binary ionic compound found to be acidic in nature. While barium carbonate has been the basic salt with the potency to be poisonous to humans.
The reaction between an acid and a base has been the neutralization reaction with the formation of salt and water. The reaction between rubidium nitrite and barium carbonate will be:
\(\rm 2\;RbNO_2\;+\;BaCO_3\;\rightarrow\;Rb_2CO_3\;+\;Ba(NO_2)_2\)
The reaction has been the displacement reaction with the tendency to displacement.
For more information about displacement reaction, refer to the link:
https://brainly.com/question/3175217
Consider the structure of caffeine (re-draw it here), and answer the following questions from a structural perspective. identify (circle on your structure) and discuss two different structural features that would account for solubility in water (hint: think intermolecular forces).
Answers
The two structural features in caffeine that account for its solubility in water are the presence of intermolecular force like hydrogen bonding and polar covalent bonds.
1. Hydrogen Bonding: Caffeine has three nitrogen atoms with lone pairs of electrons that can form hydrogen bonds with water molecules.
These hydrogen bonds increase the solubility of caffeine in water, as they allow for favorable interactions between caffeine molecules and water molecules.
2. Polar Covalent Bonds: Caffeine also contains polar covalent bonds, such as the C-O bond and N-H bond, which contribute to its solubility in water. These polar bonds create a charge separation, allowing caffeine to interact with the polar water molecules, increasing solubility.
If you look at a caffeine molecule structure, you can see the three nitrogen atoms and polar covalent bonds mentioned above.
Caffeine's solubility in water can be attributed to the presence of hydrogen bonding and polar covalent bonds within its structure. These features allow caffeine to interact favorably with water molecules, increasing its solubility in water.
For more information on intermolecular forces kindly visit to
https://brainly.com/question/29784641
#SPJ11
Please Help! Please do not give me a random, gibberish answer or I will report you and give you a low rating therefore dropping your popularity. Also, if you don't know the answer you can put your guess in the comments. I will give a Brainliest and 50 points!
What volume of solution is prepared when 25.0 g of NaCl is added to enough water to make a 2.50 M NaCl solution?
Answers
Answer:
0.171 L
Explanation:
25g NaCl * \(\frac{1 mol NaCl}{58.44g NaCl}\) * \(\frac{1 L}{2.5 mol}\)
= 0.171 L
Will mark brainliest!!

Answers
Answer:
I think its B im not sure
but i hope this helps
how many moles of H2O will be required to make 17.6 moles of O2
Answers
Answer:
1 mole
Explanation:
A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms.
How many molecules are in 2 moles of O2?
Answers
Answer:
Approx 32g
Explanation:
Oxygen has an atomic weight of 15.999. Times to is 32g
in a titration a solution of 0:100 mol l-1 h2so4 was added from a burette to 25.0ml of naoh solution. 21.4ml of the h2so4 solution was used to reach the endpoint of the titration. what was the concentration of the naoh solution?
Answers
Acetic acid and sodium hydroxide used to make a buffer solution.
What is buffer solution?
buffer solution is a waterproof solvent based solution in which consists of a mixture of containing a weak acid and the conjugate base of the weak acid, or a weak base of the solution and the conjugate acid of the weak base. They resist to a change in pH upon to the dilution or upon the addition of small amounts of a acid/alkali to them. Buffer solution resist of a change in pH when smallest amounts of a stronger acid or a stronger base are added.
Sol-Acetic acid is a weaker acid and its conjugate base is the acetate anion. That's why the addition of the strong base, hydroxide, which neutralized half of a acetic acid created a buffer and solution because we are having significant amounts of both acetic acid and its conjugate base, the acetate anion, in the solution.
To know more about buffer solution click-
https://brainly.com/question/8676275
#SPJ4
What are the three ways there can be a base change in a sequence?
Answers
Answer:
substitutions, deletions and insertions
Which process or processes produce an increase in entropy? i. n2(g) 3h2(g) right arrow. nh3(g) ii. c10h8(g) right arrow. c10h8(s) iii. ch3oh(l) right arrow. ch3oh(aq) i only ii and iii iii only i and iii
Answers
Entropy is commonly understood as the system's degree of disorder or unpredictability. The correct option is, (C) (iii) only
What is entropy?Entropy is a thermodynamic number that represents the inability of a system's thermal energy to be converted into mechanical work and is commonly understood as the system's degree of disorder or unpredictability.
The degree of unpredictability of a molecule rises when it transitions from a solid to a liquid state. As a result, the entropy will rise.
Similarly, when we move from a liquid to a gaseous state, the unpredictability of the molecule increases. As a result, the entropy will rise.
However, as we progress from gas to liquid to solid, the degree of molecular randomization reduces. As a result, the entropy will decrease.
Or, to put it another way, the higher the number of moles in a product, the higher the entropy.
(i) \(N_2(g)+3H_2(g) \rightarrow 2NH_3(g)\)
Entropy falls in this reaction because the number of moles on the product side is fewer than the number of moles on the reactant side.
(ii) \(C_{10}H_8(g) \rightarrow C_{10}H_8(s)\)
Entropy lowers in this process because the degree of randomness of the molecules diminishes as we move from a gas to a solid-state.
(iii) \(CH_3OH(l)\rightarrow CH_3OH(aq)\)
Because the methanol liquid dissociated into ions, the number of moles present on the product side is more than the number of moles present on the reactant side, entropy rises in this reaction.
Hence, the correct option is, (C) (iii) only.
Learn more about Entropy:
https://brainly.com/question/15025401
When a magnet spins within a coil of wires, what is produced?
A. blue sparks
B. glowing light
C. nuclear energy
D. an electric current
and
In an electric generator, ___ will increase the output of electricity.
A. decreasing the turns of wire
B. increasing the turns of wire
C. decreasing the size of the magnet
D. decreasing the speed if the spinning magnet
lastly
Electric field defined as force per charge. It is measured in units of newtons divided by _____.
A. watts
B. coulombs
C. mass
D. grams
thanks :)
Answers
15. What is the mass of mercury in a 250 g fish if the ppm was measured to be 0.23?
Answers
Answer: The mass of mercury will be 57.5 micrograms.
The given question deals with Analytical Chemistry where Concentration is asked here.
Concentration: Generally concentration is defined as amount of the substance per unit volume. Concentration is also expressed in parts per million(ppm) when it is a small quantity as in case of mercury content in fish.
Explanation: Given mass of the fish = 250 g,
ppm = 0.23
Now mass of mercury = 0.23 ppm × 250 g
= 0.23/1,000,000 × 250g
=0.23×250/1,000,000 g
= 0.0000575 g
=57.5 micrograms
Reference Link:https://brainly.com/question/12003074
#SPJ2
Consider the equation for the formation of water. 2h2 o2 2h2o what is the theoretical yield of h2o if 130 g of h2o is produced from 18 g of h2 and an excess of o2? 18 g 81 g 130 g 160 g
Answers
The theoretical yield of 18 gram of \(H_{2}\) is 162 gram of \(H_{2} O\) .
The amount of a product produced by a reaction is generally expressed as a percentage of the reaction's yield. The amount of product that stoichiometry predicts is known as the theoretical yield, but the amount that would be actually achieved is known as the actual yield.
The balanced chemical equation is :
\(2H_{2} +O_{2}\) → \(2H_{2} O\)
It is known that the molar mass of water is 18 gram /mol and 2 gram per mole of \(H_{2}\).
18g \(H_{2}\) (1 mol \(H_{2}\) (/2 g \(H_{2}\) ()(2 mol \(H_{2} O\) / 2 mol \(H_{2}\) ()(18 g \(H_{2} O\) /1 mol \(H_{2} O\) ) = 162 g \(H_{2} O\) .
Theoretical yield will be 162 gram.
So, the theoretical yield of 18 gram of \(H_{2}\) is 162 gram of \(H_{2} O\) .
To know more about the theoretical yield
https://brainly.com/question/14966377
#SPJ4
1d. draw a specific example (reactant, reagent and product) of the preparation of a lithium acetylide.
Answers
Lithium acetylide is an organic compound that is commonly used as a strong base in organic synthesis. It is prepared by the reaction of acetylene with lithium metal in an inert atmosphere. The reaction is exothermic and requires careful handling.
A specific example of the preparation of lithium acetylide can be illustrated by the reaction between acetylene and lithium in a dry tetrahydrofuran (THF) solvent. The reaction can be written as follows:
C₂H₂ + 2Li → Li₂C₂ + H₂
In this reaction, acetylene acts as the reactant, while lithium metal acts as the reagent. The product of the reaction is lithium acetylide, which is represented by the chemical formula Li₂C₂.
The reaction is usually carried out in an inert atmosphere, such as nitrogen or argon gas, to prevent the reaction of lithium with water or air. The solvent, THF, is used to dissolve the lithium acetylide product and to prevent the formation of side products.
The preparation of lithium acetylide is an important step in organic synthesis, as it can be used as a strong base for various reactions, such as alkylations, acylations, and reductions. The reactivity of lithium acetylide makes it a useful tool for organic chemists.
To know more about organic synthesis, refer to the link below:
https://brainly.com/question/30757552#
#SPJ11
what is the difference between porcelain and ceramic tile
Answers
Answer:
porcelain is more fragile
Explanation:
What is the organelle that builds the protein molecules using the instructions from the genes?
1 Gene version
2 nucleus
3 ribosomes
4DNA
5 Golgi Bodies
Answers
Answer : Ribosomes
the pressure inside each of two identical cylinders is equal to atmospheric pressure. one cylinder contains hydrogen, the other nitrogen. both gases are at the same temperature. the number of moles of hydrogen is greater than the number of moles of nitrogen. less than the number of moles of nitrogen. equal to the number of moles of nitrogen.
Answers
The number of moles of hydrogen is equal to the number of moles of nitrogen.
According to the ideal gas equation
PV = nRT
where
P = the pressure of the gasV = the volume of gasn = the total amount of ideal gas (moles)R = the universal gas constantT = the temperatureIn the problems there are two identical cylinders means, V₁ = V₂
The pressure inside each of two identical cylinders is equal to atmospheric pressure means, P₁ = P₂
Both gases are at the same temperature, means T₁ = T₂
\(\frac{P_1 V_1}{P_2 V_2} \:=\: \frac{n_1 RT_1}{n_2 RT_2}\)
\(\frac{P_1 V_1}{P_2 V_2} \:=\: \frac{n_1 T_1}{n_2 T_2}\)
n₁ = n₂
So the total amount of hydrogen gas is equal to total amount of ideal nitrogen gas.
Learn more about the ideal gas equation here: https://brainly.com/question/27870704
#SPJ4
Hey besties can y'all help me out
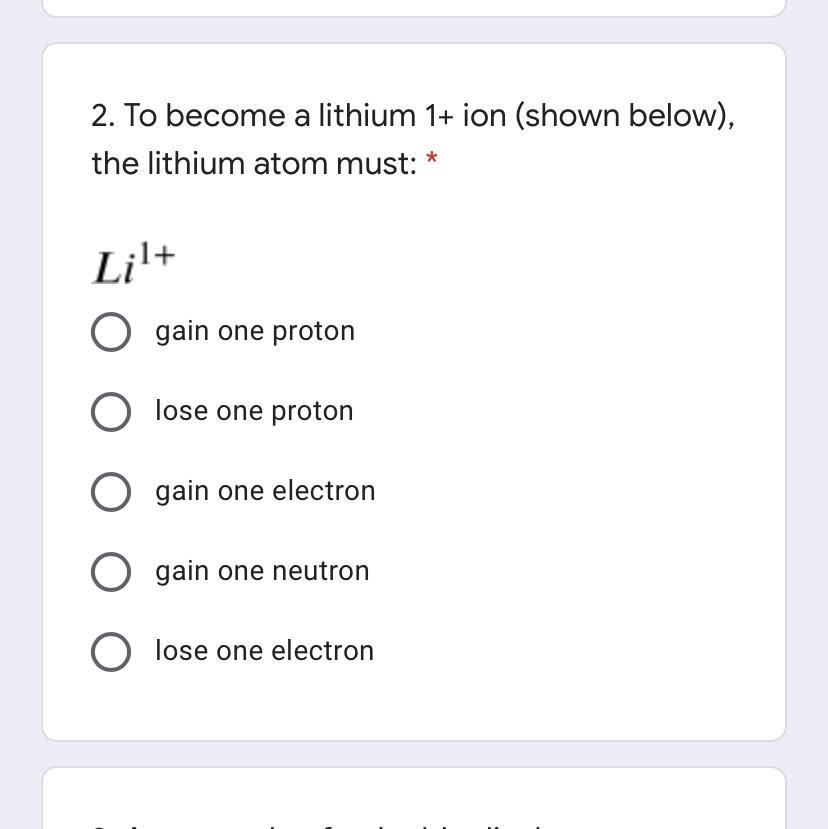
Answers
you need to lose one electron
oxidation is a example of ?the answer choices are are here
A.a financial property,B.a chemical property,C.cool science stuff,D.a physical property
Answers
Answer:
B. a chemical property
Explanation:
Oxidation requires a chemical reaction to occur, therefore it is a chemical property.
Explanation: Oxidation is the chemical process in which a substance gains oxygen or loses electrons or hydrogen.
What would happen if the amount of sodium azide used was far greater or far less thanIts 1424 grams of sodium azide which is required to produce 736 L of Nitrogen gas with the density of 1.25 g/LIts
Answers
Based on the stoichiometry of the reaction, If the mass of sodium azide used was far greater than 1424 g, the volume of nitrogen gas produced will be far greater than 736 L.
However, If the mass of sodium azide used was far less than 1424 g, the volume of nitrogen gas produced will be far less than 736 L.
What is sodium azide used for?Sodium azide is the chemical found in automobile airbags.
Automotive air bags inflate when an electrical charge triggered by automobile impact causes sodium azide, NaN3, to rapidly decompose to its component elements, nitrogen gas and sodium
The equation of the reaction is given below:
2 NaN3(s) → 2 Na(s) + 3 N2(g)According to the equation of reaction, 2 moles of sodium azide produces 3 moles of nitrogen gas.
1 mole of Nitrogen gas has a volume of 22.4 L
Molar mass of sodium azide is 65 g
Moles of sodium azide in 1424 g = 1424/65 = 21.9077 moles
21.9077 moles of sodium azide will produce 21.9077 * 3/2 moles of nitrogen gas = 32.86 moles of nitrogen gas
32.86 moles of nitrogen gas will occupy a volume of 32.86 * 22.4 = 736 L
If the mass of sodium azide used was far greater than 1424 g, the volume of nitrogen gas produced will be far greater than 736 L.
If the mass of sodium azide used was far less than 1424 g, the volume of nitrogen gas produced will be far less than 736 L.
Learn more about gas volume at: https://brainly.com/question/25736513
Which reaction occurs with a decrease in entropy?(A) N2(g) + O2(g) ® 2NO(g)(B) N2O4(g) ® 2NO2(g)(C) 2CO(g) ® C(s) + CO2(g)(D) 2HCl(aq) + Ag2CO3(s) ® 2AgCl(s) + CO2(g) + H2O(l)
Answers
The reaction that occurs with a decrease in entropy is option C, 2CO(g) → C(s) + CO₂(g).
Entropy is a measure of the disorder or randomness of a system. A decrease in entropy means a decrease in disorder, which can be achieved by a reaction that results in fewer molecules or more ordered structures. Option C, 2CO(g) ® C(s) + CO₂(g), involves the formation of a solid and a gas from two gases, which results in a decrease in the number of molecules and an increase in order.
This reaction has a negative ΔS value, indicating a decrease in entropy. In contrast, options A and B involve the formation of more molecules from fewer molecules, which results in an increase in disorder and a positive ΔS value. Option D involves the formation of more molecules from fewer molecules, but also includes the formation of a gas, which makes the ΔS value positive. Therefore, option C is the correct answer.
Learn more about entropy here:
https://brainly.com/question/13135498
#SPJ11
Given the following half-reactions: Al3+ + 3e-→ Al E° =-1.66 V Calculate the equilibrium constant at 25°C for the reaction Al3+ (aq) + 6F-(aq) 근 Alf6 3-(aq) (Enter your answer to two significant figures.) K= Submit Answer Try Another Version 10 item attem
Answers
The Nernst equation can be used to calculate the equilibrium constant of a reaction, given the half-reactions and the standard electrode potentials of the species involved in the reaction. We can use the Nernst equation to calculate the equilibrium constant (K) for the reaction below:Al3+ (aq) + 6F-(aq) → AlF63-(aq).
The half-reactions for the reaction are:Al3+ + 3e- → Al E° = -1.66 VF- → F- + e- E° = -2.87 V.
The Nernst equation is: E = E° - (RT/nF) ln(Q) where E is the electrode potential, E° is the standard electrode potential, R is the gas constant, T is the temperature in Kelvin, n is the number of electrons transferred, F is the Faraday constant, and Q is the reaction quotient.
For the reaction above: Q = [AlF63-] / ([Al3+] [F-]6) n = 6 (since 6 electrons are transferred) E°cell = E°(AlF63-) - E°(Al3+) - E°(F-)E°cell = -1.66 V - (-2.87 V) - 6(0.0257 V) * log ([AlF63-] / ([Al3+] [F-]6))E°cell = 1.21 V.
At equilibrium, Ecell = 0:0 = 1.21 V - (0.0257 V) * log (K)K = 2.12 x 1015.
Therefore, the equilibrium constant at 25°C for the reaction Al3+ (aq) + 6F-(aq) → AlF63-(aq) is K = 2.12 x 1015.
To know more about equilibrium constant visit:
https://brainly.com/question/28559466
#SPJ11
Describe what a geologist would observe if she poured acid on the sculpture
Answers
Answer:
A geologist would observe how the material that the sculpture is made out of reacts to the acid.
In lab, you calculate the density of an iron rod to be 7.30 g/cm3. The accepted value
for the density of iron is 7.80 g/cm3. What is your percent error?
Answers
Answer:
6.41 %Explanation:
The percentage error of a certain measurement can be found by using the formula
\(P(\%) = \frac{error}{actual \: \: number} \times 100\% \\ \)
From the question
actual density = 7.80 g/cm³
error = 7.30 - 7.80 = 0.5
We have
\(p(\%) = \frac{0.5}{7.8} \times 100 \\ = 6.410256...\)
We have the final answer as
6.41 %Hope this helps you
