can someone please help me, i'll give you a 5 star rating and brainliest answer

Answers
Related Questions
In the cathode ray experiment, the cathode ray is seen to move from the negative disk to the positive disk. What kind of subatomic particle will move in. the opposite direction of the cathode ray?
Answers
Answer:
Gixt8x75zurx7txr7x7tzzr7zr7z64zz7z84
A volcano erupts sending lava down its side the lava destroys the forest below which sphere is being impacted negatively? And why
PLEASE HELP ME I DONT HAVE MUCH TIME
Answers
Answer:
Biosphere
Explanation:
This is because the biosphere is the category of life. This means that plants, animals, humans, and bacteria are all apart of the biosphere.
In this chemical equation which of the following identifies the reactant(s)?

Answers
Answer:
2na+o2
Pls mark as brainliest
Answer:
A
Explanation:
2NA + 02
These are your reactants, which are always on the left side of the chemical equation.
hope this helped mark brainliest pls xx
Which of the following is not a
compound?
CO2, Chi, nací , ci
Answers
If you're talking about organic and inorganic compounds, they are all classified under one.
However, if you're talking about ionic and covalent compounds, they can be classified under them.
Carbon dioxide: Covalent
I believe you're talking about ChL, which could be considered a chemical compound or covalent.
NaCi does not exist. However, if you're talking about NaCl, which DOES exist, it is ionic.
Ci does not exist, but if it's Cl, once again, covalent.
Henceforth, I have no context. ChL OR NaCl could be considered the answer.
Why ChL? Chemical.
Why NaCl? It is the odd one out and ionic bonds are often considered a compound different from others.
how would u make a 1.0L of a 0.1 M solution of AgNO3?
Answers
Answer:
You need to dissolve 16.988 g of AgNO3 in enough water to make a final volume of 1.0 L to make a 0.1 M solution of AgNO3.
Explanation:
To make a 1.0 L of a 0.1 M solution of AgNO3, you need to know the molar mass of AgNO3, which is:
Ag = 107.87 g/mol
N = 14.01 g/mol
O = 16.00 g/mol (there are three O atoms, so 3 x 16 = 48.00 g/mol)
Total = 169.88 g/mol
Next, you need to calculate the mass of AgNO3 required to make a 0.1 M solution in 1.0 L of water:
0.1 moles/L * 1.0 L = 0.1 moles
Mass = moles x molar mass
Mass = 0.1 moles x 169.88 g/mol
Mass = 16.988 g
Therefore, you need to dissolve 16.988 g of AgNO3 in enough water to make a final volume of 1.0 L to make a 0.1 M solution of AgNO3.
Researchers stationed at different areas on a mountain and in a tunnel midway through the mountain boiled water at the same time. Even though the water at every station was at the same temperature, the pot on the top of the mountain started boiling before the others. Why?
Answers
The phenomenon observed, where water at the top of the mountain started boiling before the water at lower stations, can be attributed to the difference in atmospheric pressure at various elevations.
Atmospheric pressure decreases with increasing altitude. The pressure exerted by the atmosphere affects the boiling point of a liquid. As the pressure decreases, the boiling point of a substance also decreases.
This is because boiling occurs when the vapor pressure of the liquid equals the atmospheric pressure. When the atmospheric pressure decreases, the vapor pressure required for boiling is reached at a lower temperature.
On top of the mountain, where the atmospheric pressure is lower, the boiling point of water is lower compared to the stations at lower elevations.
Therefore, even if the water at each station was at the same initial temperature, the water at the top of the mountain reached its boiling point first because the lower atmospheric pressure allowed the vapor pressure to be achieved at a lower temperature.
In contrast, the stations located at lower elevations experience higher atmospheric pressure, requiring a higher temperature to reach the boiling point of water. Hence, the water at these stations takes longer to reach the boiling point compared to the water at the top of the mountain.
For more such questions on atmospheric pressure visit:
https://brainly.com/question/19587559
#SPJ8
What color or colors of light are transmitted by a piece of yellow glass?
A. blue and green light
B. blue and red light
C. red and green light
D. blue light only
Answers
Answer:
So combining blue with yellow light is like combining blue light with red and green light. The result of combining these three primary colors of light is to produce white light.
Explanation:
give latin name of silver
Answers
The Latin word for silver is argentum.
(will mark brainliest) pls don’t scam - due today! these are my last points please answer! please answer the first and second one
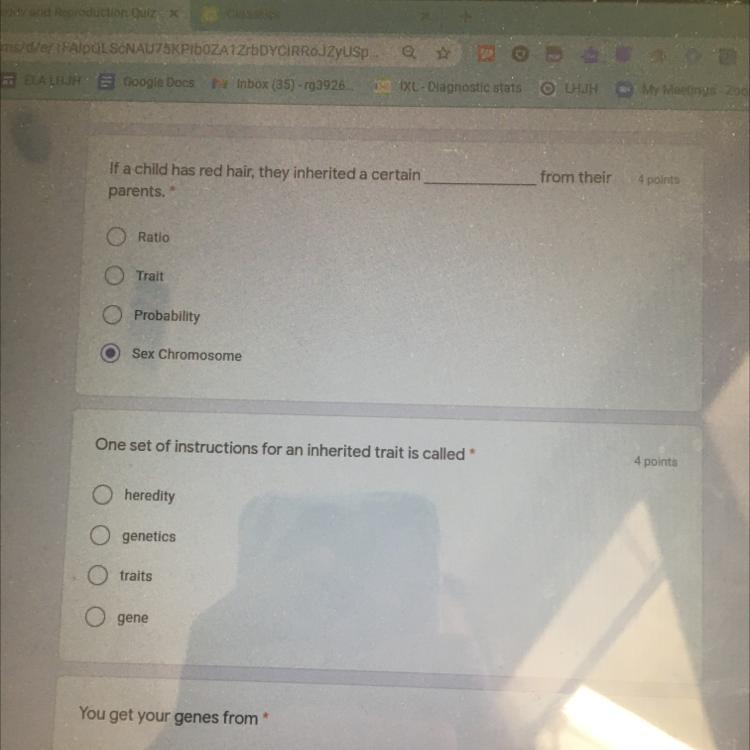
Answers
Answer:
Genetics?
Explanation:
Answer:
1. trait
2. gene
Hydrogen gas and oxygen gas are produced when sodium sulfate solution is electrolysed. Explain how oxygen gas is produced in the electrolysis of sodium sulfate solution. [4 marks
Answers
When electricity is passed through sodium sulfate solution, it separates the water molecules. Oxygen gas is produced at one electrode called the anode. At the other electrode called the cathode, hydrogen gas is produced. So, during electrolysis, oxygen gas is made at the anode and hydrogen gas is made at the cathode.
help me with this plssss


Answers
Answer:
5=D
6=B
7=A
8=?????
1A= conduction
1B= radiation
1C= convection
3: C
4: A
Explanation:
2: The heat from the hot water is been transferred along the metal handle to the other end of the spoon by the process of CONDUCTION.
Given the observed F2 data shown here from a typical three point linkage mapping cross, what is the distance between the roof and vestigial genes? Phenotype Observed Expected cinnabar, vestigial roof cinnabar, roof, vestigial wild type vestigial cinnabar, roof roof, vestigial cinnabar O 14.2 map units O 6.6map units O 13.5 map units O 7.3 map units O None of the Above
Answers
The observed F2 data shown is a typical three point linkage mapping cross. The distance between the roof and vestigial genes can be calculated based on the map units shown in the "Expected" column.
The distance between two genes on a chromosome is proportional to the frequency at which recombination occurs between them. The expected map units for the cross cinnabar, roof, vestigial to wild type is 13.5 map units, while the expected map units for the cross cinnabar, vestigial to roof is 6.6 map units. Therefore, the distance between the roof and vestigial genes is 6.9 map units (13.5 - 6.6).
The map units, also known as centimorgans (cM), are a measure of the genetic distance between two loci on a chromosome. The higher the frequency of recombination between two loci, the greater the distance between them. In this three-point linkage mapping cross, the expected map units for the cross cinnabar, roof, vestigial to wild type is 13.5 cM, while the expected map units for the cross cinnabar, vestigial to roof is 6.6 cM. By subtracting the latter from the former, we get 6.9 cM as the distance between the roof and vestigial genes.
This distance represents the average frequency of recombination between the roof and vestigial genes in the F2 population. It also indicates that there is a low probability of recombination occurring between these two genes, meaning they are likely located close to each other on the same chromosome. This information can be useful in determining the chromosomal location of these genes and in understanding their potential functional relationships.
Find out more about Phenotype Observed Expected
brainly.com/question/20566036
#SPJ4
write any three difference between shell and nucleus
Answers
Answer:
1)NUCLEUS -> It is the center of an atom which contain protons and neutrons
SHELL-> the space where the probability of finding electrons is maximum in the atom is known as shell
there are mainly four shells KLMN
2)NUCLEUS->The average potential of Nuclei is different from that of an atom.
SHELL -> The average Potential varies.
3)NUCLEUS ->Particles of the shell structure of nuclei are neutrons and protons
SHELL->Particles of the shell structure of an atom are electrons
pls rate 5 stars
ILL GIVE BRAINLY
PLEASE HELP ME
EASYYYYY
how can ionic and molecular compounds be identified using their physical and chemical properties?
Answers
As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds.
I hope it's help ;)
Chemical reactions that result in the synthesis or assembly of large molecules are referred to as: A. catabolic. B. glycolysis. C. anabolic. D. anaerobic
Answers
The chemical reactions that result in the synthesis or assembly of large molecules are referred to as anabolic.
Anabolic reactions, also known as anabolism, involve the formation of complex molecules from simpler ones. These reactions require energy and are essential for the growth and repair of cells and tissues in living organisms.. Anabolic reactions involve the synthesis or assembly of large molecules from smaller molecules, while catabolic reactions break down large molecules into smaller ones. Glycolysis is a metabolic pathway that breaks down glucose into smaller molecules, and anaerobic reactions occur without the use of oxygen.
Thus, anabolic reactions are the chemical reactions that result in the synthesis or assembly of large molecules.
To know more about anabolic reaction, click here
https://brainly.com/question/14932822
#SPJ11
Calculate the wave length of hello light emitted by sodium lamp if the frequency of the radiation is 5x10to the power of 4
Answers
6.0×10⁵m is the wavelength of hello light emitted by sodium lamp if the frequency of the radiation is 5x10⁴.
What is wavelength?A periodic wave's wavelength is its spatial period, or the length over which its form repeats. It is a property of the both traveling waves as well as standing waves in addition to different spatial wave patterns. It refers to the distance between two successive corresponding locations of the same phase just on wave, such as two nearby crests, troughs, and zero crossings.
The spatial frequency is the reciprocal of wavelength. The Greek letter lambda is frequently used to represent wavelength. The term wavelength also was occasionally used to refer to modulated waves, their sinusoidal envelopes, or waves created by the interaction of several sinusoids.
ν=c/λ
5x10⁴=3×10⁸/λ
λ =3×10⁸/ 5x10⁴
=6.0×10⁵m
Therefore, 6.0×10⁵m is the wavelength.
To know more about wavelength, here:
https://brainly.com/question/12377285
#SPJ9
the molality of the solution is 3.63 m. what quantity in moles of the unknown compound were dissolved in the solution?
Answers
The quantity of the unknown compound in moles dissolved in the solution can be calculated by multiplying the molality by the mass of the solvent.
The molality of a solution is defined as the number of moles of solute (the substance being dissolved) per kilogram of solvent (the substance doing the dissolving). It is represented by the symbol "m" and has units of mol/kg.
Given the molality of the solution as 3.63 m, we can calculate the number of moles of the unknown compound in the solution by using the equation:
n = molality x mass of solvent
Suppose the mass of the solvent is "m" kilograms. Then, the number of moles of the unknown compound can be calculated as:
n = 3.63 m x m
So, the quantity of the unknown compound in moles can be determined if the mass of the solvent is known. It is important to note that molality is a concentration unit that is independent of the volume of the solution, and is useful in cases where the density of the solution is not significantly affected by the addition of the solute.
In summary, the molality of a solution represents the number of moles of solute per kilogram of solvent, and the quantity of the unknown compound in moles can be calculated by multiplying the molality by the mass of the solvent.
To know more about molality, click here : https://brainly.com/question/30395596
#SPJ4
Which are easier for your body to metabolize: saturated or unsaturated fats?
Answers
The unsaturated fats are easier for the body to break because they are liquid at room temperature.
What are unsaturated fatty acids?Unsaturated fats, which are liquid at room temperature, are regarded as healthy fats because they help reduce inflammation and raise blood cholesterol levels. Unsaturated fatty acids that have been converted to saturated fatty acids are known as trans fats.
Conversely, saturated fats lack double bonds, while unsaturated fats do. As a result, they take on a kinkier shape and don't pack as tightly. Their bonds are more easily broken when molecules are farther apart.
Therefore, since unsaturated fats are liquid at normal temperatures, the body may more easily break them down.
To learn more about unsaturated fatty acids, refer to the link:
https://brainly.com/question/4891995
#SPJ1
what is the volume of 12 mmol of hexanol in ml? mw = 102.16 g/mol; density = 0.814 g/ml answer to at least 2 decimal places.
Answers
The volume of 12 mmol of hexanol is 14.74 ml.
What is the volume, in milliliters, of 12 mmol of hexanol?Hexanol, with a molecular weight of 102.16 g/mol, is a compound commonly used in various applications. To calculate the volume of 12 mmol of hexanol, we can use its molar mass and density.
First, we need to convert the given amount from millimoles (mmol) to moles by dividing it by 1000. So, 12 mmol becomes 0.012 moles.
Next, we can calculate the mass of 0.012 moles of hexanol by multiplying the molar mass (102.16 g/mol) by the number of moles. The result is 1.22592 grams.
To determine the volume, we divide the mass by the density of hexanol. Therefore, 1.22592 grams divided by 0.814 g/ml equals approximately 1.50799 ml.
Rounding to two decimal places, the volume of 12 mmol of hexanol is approximately 14.74 ml.
Learn more about volume
brainly.com/question/13338592
#SPJ11
what volume is occupied by5.3 mol of c02 at 28c and a pressure of .998 atm
Answers
Answer:
V = 131.23 L
Explanation:
Given data:
Moles of CO₂ = 5.3 mol
Temperature = 28°C
Pressure = 0.998 atm
Volume occupied = ?
Solution:
Formula:
PV = nRT
P= Pressure
V = volume
n = number of moles
R = general gas constant = 0.0821 atm.L/ mol.K
T = temperature in kelvin
Now we will convert the temperature.
28+273 = 301 K
0.998 atm ×V = 5.3 mol × 0.0821 atm.L/ mol.K × 301 K
V = 130.97 atm.L / 0.998 atm
V = 131.23 L
Calculate the shortest wavelength of the electromagnetic radiation emitted by the hydrogen atom in undergoing a transition from the n = 6 level.
Answers
Answer:
\(\lambda = 9.376*10^{-8} m\)
Explanation:
From the question we are told that:
\(n=6level\)
Generally the equation for Energy is mathematically given by
\(E=\frac{hc}{\lambda}\)
Since
Energy difference will be maximum when electron return to ground state
And Shortest wavelength is emitted when there is largest energy difference
Therefore
\(1/\lambda = R* (\frac{1}{nf^2} - \frac{1}{ni^2})\)
Where
\(R=Rydberg\ constant\)
\(R = 1.097*10^7\)
Therefore
\(1/\lambda = 1.097*10^7* ((\frac{1}{1^2} - \frac{1}{6^2})\)
\(\lambda = 9.376*10^{-8} m\)
Alchemy is a branch of ancient knowledge that states lead metal is a form of gold metal. Which of these best explains whether alchemy is a science or pseudoscience? It is a science because ancient knowledge is often reliable. It is a science because metallurgy is known to be a branch of science. It is pseudoscience because ancient knowledge is mystical yet reliable. It is pseudoscience because each metal is known to be a unique element.
Answers
The best statement among the following which explains whether alchemy is a science or pseudoscience is "It is pseudoscience because each metal is known to be a unique element." Hence, Option (D) is correct.
What is Alchemy ?
Alchemy began to fully evolve into chemistry in the 17th century, with a greater emphasis on rational thought and experimentation and less emphasis on spirituality and mysticism.
The alchemists were never successful in changing lead into gold, but modern nuclear physics can accomplish this task.
Therefore , The best statement among the following which explains whether alchemy is a science or pseudoscience is "It is pseudoscience because each metal is known to be a unique element." Hence, Option (D) is correct.
Learn more about Alchemy here ;
https://brainly.com/question/5997100
#SPJ2
What feature forms on the ocean floor at a divergent plate boundary where two pieces of oceanic crust are moving away from each other? One of these is found in the Atlantic Ocean. *
Mid-Ocean Ridge
Rift Valley
Trench
Glacier
Answers
Answer:
volcanos i think
Explanation:
learned it in 6th grade
the chemical agent(s) that produces highly reactive hydroxyl-free radicals and also decomposes to o2 gas is/are
Answers
The chemical agent that produces highly reactive hydroxyl-free radicals and decomposes to O2 gas is hydrogen peroxide (H2O2).
Hydrogen peroxide (H2O2) is a chemical compound that consists of two hydrogen atoms bonded to two oxygen atoms. It is known for its ability to decompose into water (H2O) and oxygen gas (O2). This decomposition process is facilitated by the presence of certain catalysts or through exposure to heat, light, or specific enzymes called catalases.
During the decomposition of hydrogen peroxide, highly reactive hydroxyl-free radicals (OH•) are generated as intermediates. These hydroxyl radicals have an unpaired electron, making them extremely reactive and capable of initiating chemical reactions with various organic and inorganic substances.
Hydroxyl radicals are powerful oxidizing agents and can react with a wide range of compounds, including pollutants, toxins, and pathogens. Their reactivity allows them to break down organic molecules, neutralize harmful substances, and contribute to processes like oxidative stress, disinfection, and wound healing.
Overall, hydrogen peroxide serves as a source of both highly reactive hydroxyl radicals and oxygen gas, playing important roles in various chemical and biological processes.
learn more about hydrogen peroxide here:
https://brainly.com/question/29102186
#SPJ11
what are electron configurations?
Answers
Answer:
In atomic physics and quantum chemistry , the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, using the notation explained below.
Explanation:
i found it on google
Answer:
To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
Explanation:
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s² 2s² 2p⁶, using the notation explained below.
A student mixes 5.0 g of a sodium bicarbonate solution with 6.0 g of a potassium aluminum sulfate solution in a sealed container. A white, solid precipitate is formed. What is the total mass after the reaction has taken place?More than 11.0 gLess than 11.0 g11.0 gNot enough information
Answers
First, we have to remember the law of conservation of matter:
"The matter can not be created nor destroyed. It can only be transformed".
Then, the answer, in theory, has to be 11.0 g, that is the addition of 5.0 g and 6.0 g.
The nucleus of a hellum atom Is Identical to:
A.a gamma particle
B.an alpha particle
C.a beta particle
D.all of the above
Answers
Answer:
B
Explanation:
Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth, produced after the planet cooled and solidified.
Answer: B. An Alpha particles
As helium is consist of 2 protons and 2 neutrons
Alpha particles, also consist of 2 protons and 2 neutrons bound together into a particle that's why identical to a helium-4 nucleus.
Why are the two equilibrium reactions below not affected by an increase in pressure?

Answers
An increase in pressure does not affect the reaction because the moles of reactants and products or the pressure of the forward reaction are equal at equilibrium.
What is chemical equilibrium?A chemical reaction is in equilibrium if the rate of the backward reaction and the forward reaction are equal.
Pressure affects the equilibrium position of chemical reactions involving gases.
For the given reactions in equilibrium, moles of reactants and products and hence, the pressure of the forward reaction are equal at equilibrium, therefore, an increase in pressure does not affect it.
Therefore, an increase in pressure will only affect the equilibrium of a reaction in which the pressure varies in the forward and backward reaction.
Learn more about pressure and equilibrium at: https://brainly.com/question/14424255
#SPJ1
Which of the following has the largest radius?
Answer:K
Answers
Answer:
the answer is k? lol thanks
Answer:
K
Explanation:
It is K
=========
Not K+ nor the other options, it is K
How has earth history affected the distribution of natural resources?
Answers
Earth's history has had a significant impact on the distribution of natural resources. Geological processes that have occurred over millions of years, such as plate tectonics, erosion, and sedimentation, have shaped the formation and distribution of various resources across the planet.
The movement of Earth's tectonic plates has created and destroyed landmasses, resulting in the formation of different types of geological deposits. For example, the collision of plates can lead to the formation of mountain ranges, which can contain valuable mineral deposits like gold, copper, and coal. The movement of plates can also create rift zones where valuable minerals like oil and gas can accumulate.
Learn more about the natural resources here.
https://brainly.com/question/20058252
#SPJ1