Answers
Answer: a
Explanation:
Answer:peas and corn
Explanation: its simple
Related Questions
How much energy (in J) is lost when a sample of iron with a mass of 28.3 g cools from 66.0 degrees celsius to 24.0 degrees celsius.
Answers
Answer:
Q = -535 J.
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the lost energy according to the following and generic heat equation:
\(Q=mC(T_F-T_i)\)
Thus, since the specific heat of iron is 0.450 in the SI units, we can plug in the mass and temperatures to obtain:
\(Q=28.3g*0.450\frac{J}{g\°C} (24.0\°C-66.0\°C)\\\\Q=-535J\)
Regards
A geologist conducts an investigation to determine the absolute age of a fossil. She then
repeats the procedure three times. Which BEST explains why she repeated the procedure
several times? (SC.6.N.1.1)
It helps her develop better procedures
It improves the accuracy of the results.
She wants all the results to be different.
She has more than one hypothesis to prove.
Answers
Answer:
Explanation:
Hehs
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
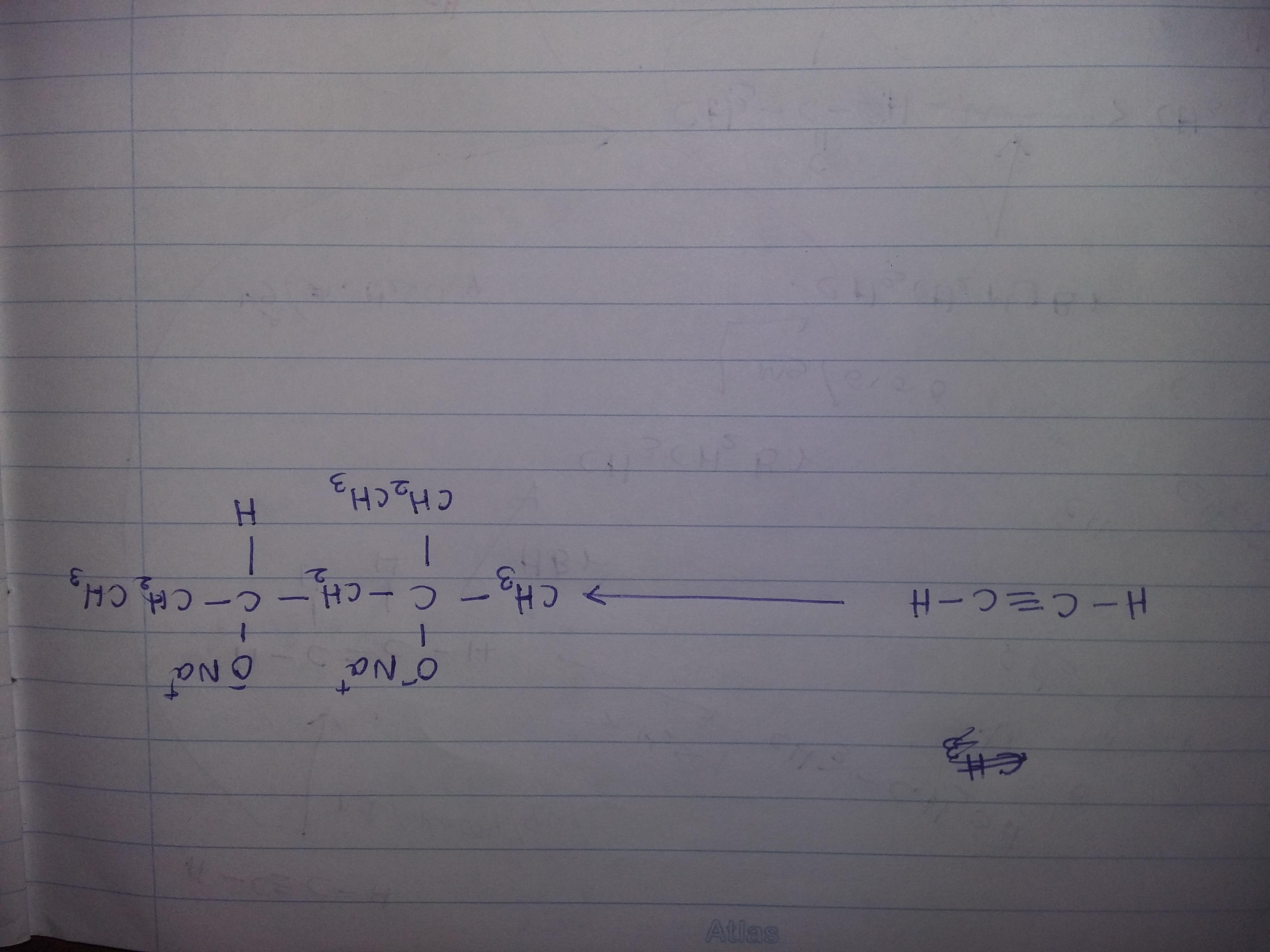
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
A liquid has rhe following properties : one-phase,colorless,boils it varying temperature. Which of the following BEST describes this liquid
A.Solution
B.substance
C.suspension
D. Coarse mixture
Answers
Answer:
Solution
Explanation:
In science, the solution is a homogeneous mixture of two or more pure substances in proportional concentrations that can be continually varied up to what has been called the solubilization limit. The word solution is usually applicable to the fluid subatomic particle, but gaseous formulations are conceivable.
Determine the limiting reactant (LR) and the mass (in g) of nitrogen that can be formed from 50.0 g N 2O 4 and 45.0 g N 2H 4. Some possibly useful molar masses are as follows: N 2O 4 = 92.02 g/mol, N 2H 4 = 32.05 g/mol.
N 2O 4( l) + 2 N 2H 4( l) → 3 N 2( g) + 4 H 2O( g)
a) LR = N2O4, 45.7 g N2 formed
b) LR = N2O4, 105 g N2 formed
c) LR = N2H4, 13.3 g N2 formed
d) LR = N2H4, 59.0 g N2 formed
e) No LR, 45.0 g N2 formed
Answers
Answer:
Option A. LR = N2O4, 45.7g N2 formed
Explanation:
The balanced equation for the reaction is given below:
N2O4(l) + 2N2H4(l) → 3N2(g) + 4H2O(g)
Next, we shall determine the masses of N2O4 and N2H4 that reacted and mass of N2 produced from the balanced equation. This is illustrated below:
Molar mass of N2O4 = 92.02 g/mol
Mass of N2O4 from the balanced equation = 1 x 92.02 = 92.02 g
Molar mass of N2H4 = 32.05 g/mol
Mass of N2H4 from the balanced equation = 2 x 32.05 = 64.1g
Molar mass of N2 = 2x14.01 = 28.02g/mol
Mass of N2 from the balanced equation = 3 x 28.02 = 84.06g
Summary:
From the balanced equation above,
92.02g of N2O4 reacted with 64.1g of N2H4 to produce 84.06g of N2.
Next, we shall determine the limiting reactant. This can be obtained as follow:
From the balanced equation above,
92.02g of N2O4 reacted with 64.1g of N2H4.
Therefore, 50g of N2O4 will react with = (50 x 64.1)/92.02 = 34.83g of N2H4.
From the calculations made above, we can see that only 34.83g out 45g of N2H4 is required to react completely with 50g of N2O4.
Therefore, N2O4 is the limiting reactant and N2H4 is the excess reactant.
Finally, we shall determine the mass of N2 produced from the reaction.
In this case the limiting reactant will be used as it will produce the maximum yield of N2 since all of it is used up in the reaction.
The limiting reactant is N2O4 and the mass N2 produced can be obtained as illustrated below:
From the balanced equation above,
92.02g of N2O4 reacted to produce 84.06g of N2.
Therefore 50g of N2O4 will react to produce = (50 x 84.06)/92.02 = 45.7g of N2.
Therefore, 45.7g of N2 were produced from the reaction.
At the end of the day,
The limiting reactant is N2O4 and 45.7g of N2 were produced from the reaction.
Which two types of information are written in an element's box in the periodic table?
I think it is B,D

Answers
Answer:
Yes it is B,D.
Explanation:
Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups.
A student would like to determine how heating a liquid changes its volume. The student hypothesizes that the liquid will increase in volume. The following list shows the steps taken by the student in order to test the hypothesis.
1.Select the liquid to test.
2.Place the liquid in a sealed container.
3.Use a Bunsen burner to heat the liquid by 10°C.
4.Measure the volume of the liquid.
5.Record the results.
What is wrong with how the student conducted the investigation?
A.
The hypothesis was not valid because it is impossible for liquids to change in volume.
B.
The volume of the liquid should be measured before it is heated.
C.
The student should have increased the temperature of the liquid by more than 10ºC.
D.
The length of time it took for the liquid to be heated should be measured.
Answers
Answer:
The volume of the liquid should be measured before it is heated.
Explanation:
Because During an experiment to test how a variable changes a substance, it is important to first observe and record the characteristics of the substance before the variable is introduced. In this case, the variable is heat energy.
make a list of information you must know in order to write the correct formula for an ionic compound (this list may require reviewing all of the activities so far).
Answers
The correct formula for an ionic compound is total charge of cations = total charge of anions.
Ionic compounds do not exist usually as molecules. In the solid state, the ionic compounds are in crystal lattice that is generally containing many ions among which each of the cation and anion. An ionic formula, such as like NaCl , is an empirical formula. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. Sodium sulfide, another ionic compound, has the formula Na2S . This formula generally indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to find the correct ratio of ions, so that we can write a correct formula.
If we know the name of a binary ionic compound, you can write its chemical formula. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Because the overall compound must be strictly electrically neutral, decide how many of each ion is needed in order for the positive and negative charges to cancel each other out.
To know about ionic compound
https://brainly.com/question/29005103
#SPJ4
what is a family in a periodic table?
Answers
Answer:
A family is a vertical column .
The elements in a family have similar chemical properties.
what is environmentally sustainable society?
Answers
Answer:
Meets the current and future basic resource needs of its people in a just and equitable manner without compromising the ability of future generations.
Explanation:
Sulfuric acid reacts with sodium carbonate to form water, a salt, and a gas. Write the formulas for the salt and other compound that are formed in the reaction.
Answers
Answer:
H2So4 + Na2Co3 -> H20 + Na2So4 + CO2
The chemical formulas for the salt and other compound that are formed in the reaction are Na₂SO₄,H₂O and CO₂.
What is chemical formula?Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
It is not the same as structural formula and does not have any information regarding structure.It does not provide any information regarding structure of molecule as obtained in structural formula.
There are four types of chemical formula:
1)empirical formula
2) structural formula
3)condensed formula
4)molecular formula
Learn more about chemical formula,here:
https://brainly.com/question/29031056
#SPJ5
The number of moles in 4.27×10^22 particles of silver is
Answers
Answer:
\(mol=0.0709mol\)
Explanation:
Hello,
In this since one mole equals 6.022x10²³ particles of silver by means of the Avogadro's number, we can compute the moles in 4.27x10²² particles as shown below:
\(mol=4.27x10^{22}particles*\frac{1mol}{6.022x10^{23}particles}\\\\mol=0.0709mol\)
Best regards.
Consider this equilibrium reaction at 400 K. Br2(g)+Cl2(g)↽−−⇀2BrCl(g)Kc=7.0 If the composition of the reaction mixture at 400 K is [BrCl]=0.00415 M, [Br2]=0.00366 M, and [Cl2]=0.000672 M, what is the reaction quotient, ???? ? ????= How is the reaction quotient related to the equilibrium constant, Kc, for this reaction?
Answers
Answer:
Q = 7.0
Q = kc. The reaction is in equilibrium
Explanation:
Based on the reaction:
Br₂ + Cl₂ ⇄ 2BrCl
Equilibrium constant of the reaction, kc, is the ratio of equilibrium concentrations products over reactants powered to its reaction coefficient:
Kc = [BrCl]² / [Br₂] [Cl₂] = 7.0
Now, reaction quotient, Q, is write as the same Kc but the concentrations are actual concentrations:
Q = [BrCl]² / [Br₂] [Cl₂]
Replacing:
Q = [0.00415M]² / [0.00366M] [0.000672M]
Q = 7.0
Now, as Q = Kc = 7.0, the reaction mixture is in equilibrium
(c) 45 g C,H, react with 45 g Cl₂ according to the equation:
Cl₂ + C6H6 C6H5Cl + HCI. What is the limiting reactant? What mass of HCI will be produced?
-
Answers
In the given reaction, the limiting reactant is C₆H₆ (benzene).
To determine the limiting reactant as well as calculate the mass of HCl produced, compare the moles of each reactant.
The number of moles for each reactant:
Molar mass of Cl₂ = 35.5 g/mol + 35.5 g/mol = 71 g/mol
Moles of Cl₂ = mass of Cl₂ / molar mass of Cl₂
= 45 g / 71 g/mol
= 0.634 moles of Cl₂
Molar mass of C₆H₆ (benzene) = 12 g/mol + 6(1 g/mol) = 78 g/mol
Moles of C₆H₆ = mass of C₆H₆ / molar mass of C₆H₆
= 45 g / 78 g/mol = 0.577 moles of C₆H₆
Determine the stoichiometry between Cl₂ and HCl:
Cl₂ + C₆H₆ → C₆H₅Cl + HCl
Here, we can see that 1 mole of Cl₂ produces 1 mole of HCl.
Thus, the limiting reactant is C₆H₆ (benzene).
Calculate the mass of HCl produced:
Molar mass of HCl = 1 g/mol + 35.5 g/mol = 36.5 g/mol
Moles of HCl produced = moles of C₆H₆ = 0.577 moles
Mass of HCl produced = moles of HCl produced × molar mass of HCl
Mass of HCl produced = 0.577 moles × 36.5 g/mol
≈ 21.04 g
Therefore, approximately 21.04 grams of HCl will be produced.
For more details regarding limiting reactant, visit:
https://brainly.com/question/10090573
#SPJ1
betrokke, onthou qrxn = AH en onthou dat "per mol" beteken om te deel.) • In an acid-
base neutralization reaction between 50.0 mL of 0.5 M nitric acid (HNO3) and 50.0
mL of 0.5 M potassium hydroxide (KOH), Arxn was found to be -2500 J. The enthalpy
change (J) per mole of base is therefore ... (Hint: determine the relevant number of
moles of base, remember Arxn = AH and remember that "per mole" means divide.)
Answers
The enthalpy tell us about the heat that may be absorbed or evolved in a reaction. The ethalpy of this reaction is -100 kJ/mol.
What is enthalpy?The term enthalpy deals with the heat that is absorbed or emitted in a reaction. The reaction equation is; HNO3 + KOH ----> KNO3 + H2O
Number of moles of HNO3 = 0.5 M * 50/1000 L = 0.025 moles
Number of moles of KOH= 0.5 M * 50/1000 L = 0.025 moles
Since the reaction is 1:1, then heat evolved = - (2500 J * 10^-3)/0.025 moles
= -100 kJ/mol
Learn more about enthalpy: https://brainly.com/question/9904853
If one photon of radiant energy supplies 3.37 × 10−19 J, then how many photons will one mole supply
Answers
One mole of radiant energy will supply 6.02 x 10^23 photons.
How can the number of photons be determined from photon energy?
The number of photons is calculated by dividing the overall energy of a pulse by the energy of one photon included inside the pulse.
One photon: What does that mean?As a result, an electromagnetic energy particle is known as a photon. Photons have no mass, thus they move at the same rate as light.
we need to first calculate the total energy in one mole of photons,
By combining the energy per photon with Avogadro's number (6.022 x 1023), one may determine the energy of a mole of photons:
Energy per mole of photons = Energy per photon x Avogadro's number
we can calculate the energy per mole of photons:
Energy per mole of photons = 3.37 × 10−19 J x 6.022 x 10^23
Energy per mole of photons = 2.03 x 10^5 J/mol
Number of photons in one mole = Energy per mole of photons / Energy per photon
Number of photons in one mole = 2.03 x 10^5 J/mol / 3.37 × 10−19 J
Number of photons in one mole = 6.02 x 10^23 photons/mol
To know more about the photon visit:
https://brainly.com/question/12015967
#SPJ1
An amateur entomologist captures a particularly excellent ladybug specimen in a plastic jar. The internal volume of the jar is 0.5L, and the air within the jar is initially at 1 atın. The bug-lover is so excited by the catch that he squeezes the jar fervently in his sweaty palm, compressing it such that the final pressure within the jar is 1.25 atm. What is the final volume of the ladybug's prison?
Answers
The final volume of the ladybug's prison is approximately 0.4 liters.
To determine the final volume of the ladybug's prison, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature. The equation for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
In this scenario, the initial volume (V1) is given as 0.5 L, and the initial pressure (P1) is 1 atm. The final pressure (P2) is 1.25 atm. We need to find the final volume (V2).
Plugging the given values into the equation, we have:
1 atm * 0.5 L = 1.25 atm * V2
Simplifying the equation, we find:
0.5 L = 1.25 atm * V2
Dividing both sides of the equation by 1.25 atm, we get:
0.5 L / 1.25 atm = V2
V2 ≈ 0.4 L
For such more questions on volume
https://brainly.com/question/31454001
#SPJ8
what is science?
its in my book name "what is science"
so explain me if you know "what is science"
Answers
Hi! Science stands for “Systematic Comprehensive Investigation and Exploration of Nature Causes and Effects”.
What is science?
Science is the systematic study of the structure and behavior of the physical natural world. It is also use for experience or can say it is an experience. Scientists are people who test science/experiments for the worlds. Science really is just the study of the nature. Science use math em empathic and logic, which are sometimes called “formal science”.
Answer:
As scientist discovered that Science is the method of learning everything about the natural world.
A hypothesis must be testable. Which hypothesis is testable? *
Ablue is the best color.
BSummer is nicer than fall.
CDogs are better than cats.
DA beagle can jump higher than a Persian cat.
Answers
the answer is D because can be tested the others ones is opinion
please mark me as brainliest
If you placed 413g of Bal2 in a beaker and filled it with water to a total volume of 750ml, calculate the molarity of the solution
Answers
To calculate the molarity of a solution, we need to determine the number of moles of the solute (Bal2) and then divide it by the volume of the solution in liters.
Given:
Mass of Bal2 = 413 g
Volume of solution = 750 ml = 0.75 L
1. Calculate the number of moles of Bal2:
First, we need to convert the mass of Bal2 to moles using its molar mass. The molar mass of Bal2 can be calculated by summing the atomic masses of boron (B) and iodine (I):
Molar mass of Bal2 = (atomic mass of B × 1) + (atomic mass of I × 2)
Molar mass of Bal2 = (10.81 g/mol × 1) + (126.90 g/mol × 2)
Molar mass of Bal2 = 10.81 g/mol + 253.80 g/mol
Molar mass of Bal2 = 264.61 g/mol
Now we can calculate the number of moles of Bal2:
Moles of Bal2 = Mass of Bal2 / Molar mass of Bal2
Moles of Bal2 = 413 g / 264.61 g/mol
Moles of Bal2 ≈ 1.561 mol
2. Calculate the molarity of the solution:
Molarity (M) = Moles of solute / Volume of solution (in liters)
Molarity (M) = 1.561 mol / 0.75 L
Molarity (M) ≈ 2.081 M
Therefore, the molarity of the solution is approximately 2.081 M.
The molarity of the solution is approximately 1.408 M as to calculate the molarity of a solution, one must need to know the number of moles of the solute and the volume of the solution in liters.
The molar mass of BaI₂ is:
Ba (barium) atomic mass = 137.33 g/mol
I (iodine) atomic mass = 126.90 g/mol
Molar mass of BaI₂ = (Ba atomic mass) + 2 × (I atomic mass)
= 137.33 + 2 × 126.90
= 137.33 + 253.80
= 391.13 g/mol
Given that the mass of BaI₂ is 413 g,
Number of moles = Mass / Molar mass
= 413 g / 391.13 g/mol
= 1.056 moles
Volume of solution = 750 ml = 750/1000 = 0.75 L
Finally, one can calculate the molarity of the solution using the formula:
Molarity = Number of moles / Volume of solution
= 1.056 moles / 0.75 L
= 1.408 M
Learn more about molarity here.
https://brainly.com/question/13386686
#SPJ1
classify each solid as a covalent, ionic, metallic, or molecular solid. drag the appropriate items to their respective bins. view available hint(s)for part a resethelp covalentdroppable ionicdroppable metallicdroppable moleculardroppable
Answers
The appropriate items to their respective bins are:
Covalent: Carbon dioxide
Ionic: Table salt
Metallic: Iron
Molecular: Sugar
What is bins?
Bins are containers used for storing and organizing items. They can be made from a variety of materials, including wood, metal, and plastic, and come in many shapes and sizes. Bins are often used in large warehouses, retail stores, and other business establishments to store and organize products, tools, and other items. In the home, bins can be used to store toys, shoes, and other items that need to be kept organized.
To learn more about bins
https://brainly.com/question/29634744
#SPJ4
A gas has a volume of 3.7 liters with a pressure of 1.75 atm. What is the pressure of the gas if its volume is raised to 4.5 L?
Answers
Answer: 251 K
Explanation: hope this helps :)
How much energy is required to raise the temperature of 4.0 g of mercury metal from 9.3 oC to 83.0 oC.
Answers
From the specific heat capacity of mercury, the amount of heat energy required to raise the temperature of 4.0 g of mercury metal from 9.3 °C to 83.0 °C is 77.792 J.
What is the specific capacity of mercury?The specific heat capacity of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius or kelvin.
The specific heat capacity of a substance is a constant that can be used to calculate the amount of heat required to raise the temperature of a given mass of a substance to any temperature.
The specific heat capacity of mercury is 0.140 J/g/k.
The formula for calculating specific heat capacity is given below:
Specific heat capacity, c = Δq/mΔT
where;
Δq = heat change
m = mass of the substance
ΔT = temperature change
The Heat required, Δq, will then be:
Δq = m * c * ΔT
Heat required, Δq = 4.0 * 0.140 * (83.0 - 9.3)
Heat required, Δq = 77.792 J
Lear more about specific heat capacity at: https://brainly.com/question/26866234
#SPJ1
True or false. An ion is an electrically neutral atom that has the same number of protons and neutrons
Answers
Answer:
False
Explanation:
Atoms are neutral Ions carry a charge. Take a look at the groups in the periodic table for extra help in understanding this.
Use the Debye–Hückel equation to calculate the activity coefficient (
Answers
Answer:
log = ( - 0.51 z 2) / ( 1 + ( / 305)
Explanation:
What is the Lewis structure for *OPCl3 and AlCl6^3-? What are their electron/molecular geometry and Ideal Bond Angle ?
Answers
Answer:
Here's what I get
Explanation:
1. POCl₃
(a) Lewis structure
Set P as the central atom, with O and Cl atoms directly attached to it.
Electrons available = P + O + 3Cl = 5 + 6 + 3×7 = 11 + 21 = 32
Arrange these electrons to give every atom an octet. Put a double bond between P and O.
You get the structure shown below.
(b) Geometry
There are four bond pairs and no lone pairs about the P atom.
Electron pair geometry — tetrahedral
Molecular geometry — tetrahedral
(c) Ideal bond angles
Tetrahedral bond angle = 109.5°
2. AlCl₆³⁻
(a) Lewis structure
Set Al as the central atom, with the Cl atoms directly attached to it.
Electrons available = Al + 6Cl + 3(-) = 3 + 6×6 +3 = 6 + 36 = 42
Arrange these electrons to give every atom an octet. Assign formal charges.
You get the structure shown below.
(b) Geometry
There are six bond pairs and no lone pairs about the Al.
Electron pair geometry — octahedral
Molecular geometry — octahedral
(c) Ideal bond angles
Axial-equatorial = 90°
Equatorial-equatorial = 120°
Axial-axial = 180°


Afarmer applies 1705 kg of a fertilizer that contains 10.0% nitrogen to his fields each year. Fifteen percent (15.0%) of the fertilizer washes into a river
that runs through the farm.
5th attempt
Il See Periodic Table See Hint
If the river flows at an average rate of 0.455 cubic feet per second, what is the additional concentration of nitrogen (expressed in
milligrams of nitrogen per liter) in the river water due to the farmer's fertilizer each year?
mg/L
Answers
Answer:
The additional concentration of nitrogen in the river water due to the farmer's fertilizer is 0.0629 milligrams per liter
Explanation:
The mass of the fertilizer the farmer applies = 1705 kg
The percentage of nitrogen contained in the fertilizer = 10%
Therefore;
The mass of nitrogen contained in the fertilizer = 10% of 1705 kg = 10/100 × 1705 kg
The mass of nitrogen contained in the fertilizer = 10/100 × 1705 kg = 170.5 kg
The percentage of the fertilizer that washes into the river that runs through the farm = 15.0%
The mass of the fertilizer that washes into the river = 15/100 × 170.5 = 25.575 kg = 25575 g = 25575000 mg
The volume of water that flows through the farm = 0.455 cubic feet per second
The volume of water that flows through the farm in a year = 0.455 ft³ × 60 × 60 × 24 × 365 = 14348880 ft³ = 406,315.03 m³ = 406,315,034 l
The number of moles of nitrogen = Mass of nitrogen/(Molar mass of nitrogen)
Molar mass of nitrogen =14.0067 g/mol
Therefore;
The number of moles of nitrogen = 25575 kg/(14.0067 g/mol) = 1.825.912 moles
The additional concentration of the nitrogen in moles = 1.825.912 moles/(406,315,034 l) = 4.49 × 10⁻⁶ mol/liter
The additional concentration of the nitrogen in milligrams of nitrogen per liter of water = 25575000/406,315,034 = 0.0629 milligrams of nitrogen per liter.
Therefore, the additional concentration of nitrogen in the river water due to the farmer's fertilizer = 0.0629 milligrams per liter
PLEASE HELP ME!!!!!!!

Answers
Answer:
The heat capacity of the metal underneath the gold is 0.431 J/g°C
Explanation:
Using the formula as outlined in the image:
Q = m × c × ∆T
Where;
Q = amount of heat energy (J)
m = mass of substance (g)
c = specific heat capacity (J/g°C)
∆T = change in temperature (°C)
According to the information in this question;
Q = 503.9J
m = 23.02g
c = ?
∆T = 74°C - 23.2°C = 50.8°C
Using Q = m × c × ∆T
c = Q ÷ m∆T
c = 503.9 ÷ (23.02 × 50.8)
c = 503.9 ÷ 1169.42
c = 0.431 J/g°C
From the above heat capacity of the metal underneath the gold, it is obvious that the metal is not pure gold (c = 0.129J/g°C)
A gas takes
up a volume of 17 L, has a
pressure of 2.3 atm, and a temperature
of 299 K. If I raise the temperature to
350 K and lower the pressure to 1140
mmHg, what is the new volume?
Answers
Answer:
V₂ = 25.065 L
Explanation:
Given data:
Initial volume = 15L
Initial pressure = 1500 mmHg (1500/760 = 1.97 atm)
Initial temperature = 299 K
Final temperature = 350 K
Final volume = ?
Final pressure = 1050 mmHg (1050/760 = 1.38 atm)
Formula:
P₁V₁/T₁ = P₂V₂/T₂
P₁ = Initial pressure
V₁ = Initial volume
T₁ = Initial temperature
P₂ = Final pressure
V₂ = Final volume
T₂ = Final temperature
Solution:
V₂ = P₁V₁ T₂/ T₁ P₂
V₂ = 1.97 atm × 15 L × 350 K / 299 K × 1.38 atm
V₂ = 10342.5 atm .L. K / 412.62 K.atm
V₂ = 25.065 L
Is Fluorine is considered a molecular compound?
Answers
fluorine is an element on it's own so no
(Answer) Fluorine is considered a molecular element
(Extra) A molecule is a substance that contains two or more atoms of the same element or different elements that are chemically bounded together and can take part in a chemical reaction.
