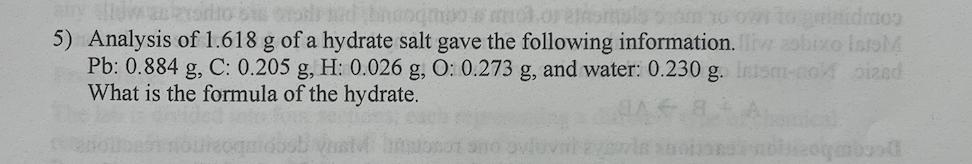Answers
The formula of the hydrated salt is Pb(C₂H₃O₂)₂.H₂O.
What is the formula of the hydrated salt?To determine the formula of the hydrated salt, we need to find the molar ratio between the salt and the water molecules in the compound.
First, we can calculate the number of moles of each element present in the sample:
moles of Pb = 0.884 g / 207.2 g/mol = 0.00426 mol
moles of C = 0.205 g / 12.01 g/mol = 0.0171 mol
moles of H = 0.026 g / 1.008 g/mol = 0.0258 mol
moles of O = 0.273 g / 16.00 g/mol = 0.0171 mol
moles of water = 0.230 g / 18.02 g/mol = 0.0128 mol
Next, we can find the simplest molar ratio between the salt and the water. To do this, we divide the number of moles of each element by the smallest number of moles, which is 0.0128 mol:
moles of Pb: 0.00426 mol / 0.0128 mol = 0.332
moles of C: 0.0171 mol / 0.0128 mol = 1.33
moles of H: 0.0258 mol / 0.0128 mol = 2.02
moles of O: 0.0171 mol / 0.0128 mol = 1.33
moles of water: 0.0128 mol / 0.0128 mol = 1
The molar ratio between the salt and water is 1:1, so the formula of the hydrated salt is Pb(C₂H₃O₂)₂.H₂O.
Learn more about hydrated salt formula at: https://brainly.com/question/28106636
#SPJ1
Related Questions
Even though plutonium−239 (t1/2 = 2.41 × 104 yr) is one of the main fission fuels, it is still a radiation hazard present in spent uranium fuel from nuclear power plants. How many years does it take for 95% of the plutonium−239 in spent fuel to decay?
Answers
It takes about 1.41 × 10⁵ years for 95% of the Plutonium-239 (Pu-239) in spent fuel to decay.
To calculate the time it takes for 95% of Plutonium-239 (Pu-239) to decay, we can use the formula for radioactive decay:
\(N_{t} =N_{o}\) × e^(-λt)
where \(N_{t}\) is the amount of radioactive material remaining after time t, \(N_{o}\) is the initial amount of radioactive material, λ is the decay constant, and e is the mathematical constant equal to about 2.718.
We can solve for t when \(N_{t}\) is 0.05N0 (95% decay) and substitute the values for λ and the half-life of Pu-239:
0.05N0 = N × \(e^{(-0.693/t1/2 * t)}\)
0.05 = \(e^{(-0.693/2.41 * 10^4 * t)}\)
ln(0.05) = -0.693/2.41 × 10⁴ × t
t = 1.41 × 10⁵ years
To learn more about Plutonium follow the link:
https://brainly.com/question/8052332
#SPJ1
For each of these molecules, identify the proper MO diagram and the number of valence electrons. The 1
orbital is not shown.
Identify the MO diagram for B2.
diagram A
diagram B
B2
valence e−:
6
Identify the MO diagram for C2.
diagram A
diagram B
C2
valence e−:
6
Identify the MO diagram for N2.
diagram A
diagram B
N2
valence e−:
10
TOOLS
x10y
Identify the MO diagram for O2.
diagram B
diagram A
O2
valence e−:
16
Identify the MO diagram for F2
.
diagram B
diagram A
F2
valence e−
Answers
The molecular orbitals for each of the required molecules has been shown in the images attached.
What is an MO diagram?
An illustration of a molecule's molecular orbitals and the corresponding energy levels is called a molecular orbital (MO) diagram. MO diagrams are used to illustrate how the atomic orbitals in a molecule combine to generate the molecular orbitals.
The diagram shows the internuclear distance between the two atoms on the horizontal axis and the relative energy of the atomic orbitals on the vertical axis.
Learn more about MO diagram:https://brainly.com/question/30389469
#SPJ1





2. Consider the combustion of ethylene,
C₂Ha(g) + 3 O₂(g) → → 2 CO2(g) + 2 H₂O(g)
a)If the concentration of C₂H4 is decreasing at the rate of 0.036 M/s, what are the rates of change
in the concentrations of CO₂ and H₂O?
b) Smol C₂H4 is placed in a 2.0L container, after 1minute, 2mols of C₂H4 remained. What is the
rate of consumption of C₂H4? What is the rate of O₂ in the reaction?
Answers
(a). The rate of change in the concentration of \(H_{2}O\) and the rate of change in the concentration of \(CO_{2}\) is: 0.072 M/s.
(b). The rate of consumption of \(O_{2}\) is: 0.10 mol \(O_{2}\) per second.
What is concentration?
a) To determine the rates of change in the concentrations of \(CO_{2}\) and \(H_{2}O\) , we first need to determine the stoichiometric coefficients of each reactant and product in the balanced chemical equation.
From the balanced chemical equation:
1 mol \(C_{2}H_{4}\) reacts to form 2 mol \(CO_{2}\) and 2 mol \(H_{2}O\).
Therefore, the rate of change in the concentration of \(CO_{2}\) is:
(0.036 M/s) x (2 mol \(CO_{2}\) /1 mol \(C_{2}H_{4}\)) = 0.072 M/s
The rate of change in the concentration of \(H_{2}O\) is also:
(0.036 M/s) x (2 mol \(H_{2}O\) /1 mol \(C_{2}H_{4}\)) = 0.072 M/s
What is consumption?
b) To find the rate of consumption of \(C_{2}H_{4}\), we can use the formula:
rate = Δ[ \(C_{2}H_{4}\)]/Δt
Initially, the concentration of \(C_{2}H_{4}\) is:
n/V = 2 mol / 2.0 L = 1.0 M
After 1 minute, the concentration of \(C_{2}H_{4}\) is:
n/V = 2 mol / 2.0 L = 1.0 M
(change in concentration is 0)
Therefore, the rate of consumption of \(C_{2}H_{4}\) is:
rate = Δ[ \(C_{2}H_{4}\)]/Δt = (1.0 M - 1.0 M) / 60 s = 0 M/s
The rate of \(O_{2}\) consumption can be found by using the stoichiometric ratio between \(C_{2}H_{4}\) and \(O_{2}\) in the balanced chemical equation:
1 mol \(C_{2}H_{4}\) reacts with 3 mol \(O_{2}\) .
Initially, we have 6 mol \(O_{2}\) in the container.
After 1 minute, 2 mol \(C_{2}H_{4}\) are consumed, which corresponds to the consumption of 6 mol \(O_{2}\) :
6 mol \(O_{2}\) / 2 mol \(C_{2}H_{4}\) = 3 mol \(O_{2}\) / 1 mol \(C_{2}H_{4}\)
Therefore, the rate of consumption of \(O_{2}\) is:
rate = (3 mol \(O_{2}\) / 1 mol \(C_{2}H_{4}\)) x (0.0333 mol \(C_{2}H_{4}\)/s) = 0.10 mol \(O_{2}\) per second.
To know more about consumption rate, visit:
https://brainly.com/question/14269017
#SPJ9
When a soda is poured into a glass and the soda bubbles, is it the result of a chemical change? Explain your answer.
Answers
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
What expiriment is most likely to take the longest to change colour The Iodine Clock ReactionYou will study the rate of reduction of potassium persulfide (K2S2O8) by sodium iodide (NaI). The net ionic equation for this reaction is the following:S2O82–+ 2 I - → 2 SO42– + I2
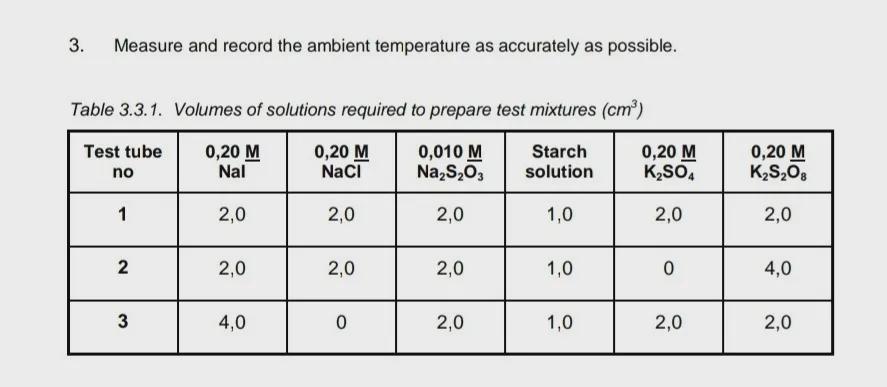
Answers
Chemistry => Kinetics => Reaction Rate
The reaction rate tells us how fast the reactants are used up, or in other words, the speed with which products are produced.
The color change in this type of reaction is due to the formation of products. The rate at which they are produced depends on several factors, including the number of moles of products and reactants.
The longest will be the one with the fewest moles of reactants. The longest will be the one with the fewest moles of reactants and the greatest number of reactants.
We have as reactants the following compounds: NaI and K2S2O8.
Experiments 2 and 3 have a major mole of reactants. Experiment 2 has a greater number of moles of K2S2O8. Experiment 3 has a greater number of moles of NaI.
Experiment 1 has less number of moles of the reactants, which means that the reaction rate of this experiment will be lower and it will take the longest to change color.
Therefore, the answer will be: Experiment 1
NEED HELP ASAP PLEASE HELP

Answers
Answer:
I think it's A not D or C maybe B probably A
What type of words should you include when you write your hypothesis
Answers
Answer:
An if/then statement. If ____happens, then _____ happens.
Explanation:
About Energy Transformation (Such as mechanical energy, chemical energy, electric energy, thermal energy)
Please complete the chart:
Objects are: Flashlight, Chocolate, Gas, Dynamite, Olympic diver on platform, Match, Stretched elastic band
What to do to release the stored energy of the objects and for each object what is the energy transformed into?

Answers
Object Action to Release Energy Energy Transformation
Flashlight Press the switch Electric Energy → Light Energy
Chocolate Consume or melt Chemical Energy → Thermal Energy
Gas Ignite or burn Chemical Energy → Thermal Energy
Dynamite Detonate Chemical Energy → Mechanical Energy + Thermal Energy + Sound Energy + Light Energy
Olympic diver Jump or dive Potential Energy (Gravitational) → Kinetic Energy (Mechanical)
on platform
Match Strike against a rough surface Chemical Energy → Thermal Energy + Light Energy
Stretched elastic band Release one end Elastic Potential Energy → Kinetic Energy (Mechanical)
Flashlight: To release the stored energy in a flashlight, you need to press the switch. This action completes an electrical circuit, allowing the electric energy stored in the battery to flow through the bulb, transforming into light energy.
Chocolate: Consuming or melting chocolate releases its stored energy. When you eat chocolate, it undergoes a chemical reaction in your body, breaking down the complex molecules and converting the chemical energy stored in the chocolate into thermal energy, providing you with warmth.
Gas: The stored energy in gas can be released by igniting or burning it. When gas reacts with oxygen in the presence of heat or a flame, a chemical reaction occurs, converting the chemical energy stored in the gas into thermal energy and producing light and heat.
Dynamite: To release the stored energy in dynamite, it needs to be detonated. When detonated, the chemical energy stored in dynamite rapidly transforms into various forms of energy, including mechanical energy (shockwave and debris movement), thermal energy (from the explosion heat), sound energy (from the blast), and light energy (from the explosion flash).
Olympic diver on platform: The stored energy in an Olympic diver on a platform is gravitational potential energy. To release this energy, the diver needs to jump or dive off the platform, converting the potential energy into kinetic energy as they descend, eventually entering the water.
Match: To release the stored energy in a match, you need to strike it against a rough surface. This action causes a chemical reaction in the match head, converting the chemical energy stored in the match into thermal energy and light energy, resulting in a flame.
Stretched elastic band: Releasing one end of a stretched elastic band allows it to return to its original shape, converting the stored elastic potential energy into kinetic energy. As the elastic band snaps back, it moves and vibrates, exhibiting mechanical energy.
These examples demonstrate different energy transformations, including chemical energy being converted to thermal energy, electrical energy transformed into light energy, potential energy being converted to kinetic energy, and elastic potential energy being converted to mechanical energy.
For more such questions on Energy Transformation
https://brainly.com/question/20735045
#SPJ11
predict whether an atom of argon will be most strongly attracted to another atom of argon, an atom of neon, or an atom of krypton.
Answers
Dispersive forces act on intermolecular interactions between Ar and Ar, Ne, and Kr.
define atom ?
Every atom is made up of a nucleus and one or more electrons that are attached to the nucleus. One or more protons and a number of neutrons make up the nucleus. Only the most prevalent kind of hydrogen is neutron-free.
Every solid, liquid, gas, and plasma is made up of atoms that are either neutral or ionised. Atoms are incredibly tiny, measuring around 100 picometers across. Because they are so tiny, it is impossible to precisely anticipate their behaviour using classical physics, as if they were tennis balls, due to quantum phenomena.
The nucleus contains more than 99.94% of an atom's mass. Protons have a positive electric charge, electrons have a negative electric charge, and neutrons have none.
Dispersive forces act on intermolecular interactions between Ar and Ar, Ne, and Kr
Because their other electrons are placed in shells further from the nucleus, heavier atoms interact more strongly than lighter atoms because they are more easily deformed by external influences.
To learn more about atoms follow the given link: https://brainly.com/question/2079874
#SPJ4
A mixture of 14.0 grams of hydrogen, 84.0 grams of nitrogen, and 2.0 moles of oxygen are placed in a flask. When the partial pressure of the oxygen is 78.00 mm of mercury, what is the total pressure in the flask?
Answers
Total pressure in the flask : 468 mmHg
Further explanationDalton's law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases
Can be formulated:
P tot = P1 + P2 + P3 ....
The partial pressure is the pressure of each gas in a mixture
For partial gas :
Pgas₁=x₁.P tot
x₁ = mole fraction of gas 1
mol of each gas :
H₂ = 14 g : 2 g/mol= 7 moles
N₂ = 84 g : 28 g/mol = 3 moles
O₂ = 2 moles
Total moles = 7+3+2= 12 moles
Partial pressure of O₂ :
\(\tt P_{O_2}=x_{0_2}\times P_{tot}\\\\78~mmHg=\dfrac{2}{12}\times P_{tot}\\\\P_{tot}=\dfrac{78\times 12}{2}=468~mmHg\)
The study of chemicals is called chemistry.
The correct answer is 468mmhg.
Dalton's law defined as partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases
The formula is stated as follows:-
\(P_{total} = P_1 + P_2 + P_3...\)
The partial pressure is the pressure of each gas in a mixture. For partial gas is as follows :
\(P_{gas}_1=X_1.P_{total}\)
The \(X_1\) defined as a mole fraction of gas 1
The mole of each gas is as follows:-
\(H_2 = \frac{14}{2}= 7 molesN_2 = \frac{84}{28} = 3 molesO-_2 = 2 moles\)
Total moles = \(7+3+2= 12 moles\)
The partial pressure \(O_2\) is as follows:-
\(P_{total} = \frac{78*12}{2} =468mm\).
Hence, the correct answer is 468mmhg.
For more information, refer to the link:-
https://brainly.com/question/25305623
What is the energy of a photon of wavelength 5.50 x 10(-7) m in Joules?
Answers
The answer is 1.09 x \(10^{-35}\) J
To find the energy of a photon with a given wavelength,
Substitute the wavelength 5.50 x 10(-7)Planck's constant in joules (h = 6.6261 1034 J/s), The speed of light (c = 299792458 m/s) into equationE = h x c x λ
You'll obtain an energy result in joules using these units (J).
According to the question,
wavelength, λ = 5.50 x \(10^{-7}\)
speed of light, c = 3 x \(10^{8}\) m/s
Planck's constant, h = 6.62607015 x \(10^{-34}\)
E = 6.62607015 x \(10^{-34}\) x 3 x \(10^{8}\) x 5.50 x \(10^{-7}\)
E = 1.09 x \(10^{-35}\) J
Therefore, the energy of a photon is 1.09 x \(10^{-35}\) J
To know more about the energy of photons:
brainly.com/question/16387660
How many moles are in 33 grams of Boron (B)?
Answers
Answer:
2.77495 moles
Explanation:
Boron= 10.811g (according to periodic table)
\(33g*\frac{1mole}{10.811g B}=2.774951 moles B\)
Which of the following is not the same as 1,400 mL? a. 1.4 cm³ b 1.4 L c. 1,400 cm³ d. 140 cL
Answers
answer should be 1.4 cm³
1 L = 10 and so
dL = 100 and then
cL = 1,000
mL = 0.001 m³
1 m³ = 1,000
dm³ = 1,000,000
cm³ = 1,000,000,000
mm³ = 1,000 L
So, 1 mL = 1 cm³ = 0.001 L = 0.1 cL
1,400 mL = 1,400 cm³ = 1.4 L = 140 cL
Answer:
1.4 cm^3
Explanation:
What is the mass percent of calcium chloride if 57 g of CaCl2 is
dissolved in 334 g of water?
Answers
Answer:
Solubility in water Anhydrous: 74.5 g/100 mL (20 °C) Hexahydrate: 49.4 g/100 mL (−25 °C) 59.5 g/100 mL (0 °C) 65 g/100 mL (10 °C) 81.1 g/100 mL (25 °C) 102.2 g/100 mL (30.2 °C) α-Tetrahydrate: 90.8 g/100 mL (20 °C) 114.4 g/100 mL (40 °C) Dihydrate: 134.5 g/100 mL (60 °C) 152.4 g/100 mL (100 °C)
In the laboratory you dissolve 19.4 g of sodium chloride in a volumetric flask
and add water to a total volume of 375 mL.
What is the molarity of the solution? In M. What is the concentration of the sodium cation? In M. What is the concentration of the chloride anion? In M.
Answers
Answer: 0.885 M
Explanation: 19.4 grams of NaCl is (19.4g/58.55 g/mole) = 0.332 moles of NaCl. 375ml is 0.375 liters. Molar is moles per liter: (0.332 moles NaCl/0.375 liters) = 0.885 M
Determine whether the following five molecules are polar or nonpolar and explain your answer:
a) Beryllium chloride b) Hydrogen sulphide c) Sulphur trioxide d) Water e) Trichloromethane
Answers
The following are categorized into polar or nonpolar molecules:
a) Beryllium chloride - nonpolar b) Hydrogen sulphide - polar c) Sulphur trioxide - nonpolar d) Water - polar e) Trichloromethane - polar How to determine polar or nonpolar?a) Beryllium chloride (BeCl₂) is a nonpolar molecule. The Be-Cl bond is polar due to the electronegativity difference between beryllium and chlorine, but the molecule is linear with the two polar bonds pointing in opposite directions, resulting in a net dipole moment of zero.
b) Hydrogen sulphide (H₂S) is a polar molecule. The H-S bond is polar due to the electronegativity difference between hydrogen and sulfur, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
c) Sulphur trioxide (SO₃) is a nonpolar molecule. The S-O bonds are polar due to the electronegativity difference between sulfur and oxygen, but the molecule is trigonal planar with the three polar bonds pointing in different directions, resulting in a net dipole moment of zero.
d) Water (H₂O) is a polar molecule. The H-O bond is polar due to the electronegativity difference between hydrogen and oxygen, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
e) Trichloromethane (CHCl₃) is a polar molecule. The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine, and the molecule has a tetrahedral shape, resulting in a net dipole moment that is not zero.
Find out more on polar or nonpolar here: https://brainly.com/question/17118815
#SPJ1
A titration required 42.00 mL of 0.150 M NaOH. How many moles of NaOH is this?
Answers
Answer:
0.006342moles
Explanation:
1000ml of NaOH contain 0.151moles
42ml of NaOH contain (42*0.151)/1000 moles
=0.006342moles
how many watts of power does a model rocket engine have if it does 66J of work on a model rocket in 0.9 seconds
Answers
Answer:
Power developed by a model rocket engine will be 73.3 watts. Power is the ratio of the work done or the enrgy to the time period.
What is the power output?
The rate of the work done is called the power output. It is denoted by P.Its unit of a watt.
E is the energy = 66 J
P is the power output =?
t is the time period = 0.9 sec
The power output is given as;
p = E
t
p = 66
0.9
P=73.3 watt
Hence power developed by a model rocket engine will be 73.3 watts.
what is a metallic bond
Answers
Answer:
A metallic bond is the force of attraction between a positively charged metal ion and the valence electrons it shares with other ions of the metal.
Explanation:
Hope this helps :)
Answer:
Metallic bond, force that holds atoms together in a metallic substance. Such a solid consists of closely packed atoms. In most cases, the outermost electron shell of each of the metal atoms overlaps with a large number of neighboring atoms.
Explanation:
If a solution's hydronium ion concentration is 1.0E-10 what is it's hydroxide ion concentration?Express your answer in Scientific notation using the format where 1.0 x 102 would be written as 1.0E2 no spaces allowed
Answers
The answer is [OH-] = 1.0E-4
During the decomposition of KClO3, 2.94 grams of oxygen gas are created. How many moles of KClO3 reacted? Report your answer with 3 significant figures.
Answers
The balanced chemical equation for KClO3 decomposition is: 2KClO3(s) → 2KCl(s) + 3O2(g). 0.061 moles of KClO3 reacted. Rounded to 3 significant figures, the answer is 0.0610 moles of KClO3.
How many moles of KClO3 reacted?
The balanced chemical equation for KClO3 decomposition is:
2KClO3(s) → 2KCl(s) + 3O2(g)
According to the equation, for every 3 moles of O2 produced, 2 moles of KClO3 react. Therefore, the number of moles of KClO3 can be calculated as:
moles of KClO3 = (2/3) × moles of O2
To find the moles of O2 produced, we need to use the molar mass of O2, which is 32 g/mol. As a result, the number of moles of O2 is:
moles of O2 = mass of(O2) / molar mass(O2)
moles of O2 = 2.94 g / 32 g/mol
moles of O2 = 0.092 mol
Now we can calculate the moles of KClO3:
moles of KClO3 = (2/3) × moles of O2
moles of KClO3 = (2/3) × 0.092 mol
moles of KClO3 = 0.061 mol
Therefore, 0.061 moles of KClO3 reacted. Rounded to 3 significant figures, the answer is 0.0610 moles of KClO3.
To learn more about chemical equation, visit: https://brainly.com/question/29886207
#SPJ1
A scientist added bacteria and a nutrient medium that could support the growth of the bacteria to a sterilized petri dish. No other materials were added. The graph models the growth of the bacteria over time.
Answers
Answer:
A scientist added bacteria and a nutrient medium that could support the growth of the bacteria to a sterilized petri dish. No other materials were added. The graph models growth of the bacteria over time. ... The bacteria did not reproduce in the system, and eventually each individual died at the end of its life cycle.
Explanation:
Plz mark brainliest thanks
Given the reaction at equilibrium:
2NO2(g) → N204(g) Heat of
reaction is -55.3 kJ) What type of
reaction is this?
O Endothermic
O Exothermic
Answers
When the equilibrium constant is higher than one, it indicates that the reaction prefers to produce products, whereas if the equilibrium constant is less than one, it indicates that the reaction prefers to produce reactants. If the equilibrium constant is equal to one, the reaction proceeds in both directions equally.
In a chemical reaction, exothermic reactions are defined as reactions that release heat into their environment. It implies that heat is given off when reactants are converted to products. At equilibrium, an exothermic reaction continues to be exothermic, meaning that heat is given off even after the reaction reaches a state of equilibrium.There are two types of reactions: exothermic and endothermic.
A reaction is classified as exothermic if it releases heat, and endothermic if it absorbs heat. The direction of the reaction is determined by whether it is exothermic or endothermic. At equilibrium, the reaction is no longer moving forwards or backwards. It's also worth noting that reactions can be exothermic in one direction and endothermic in the other.
The equilibrium constant (K) is defined as the ratio of the concentration of products to the concentration of reactants in the chemical reaction equation. It is used to express how much of the products is generated by the reaction in comparison to the reactants. the equilibrium constant aids in the identification of the direction in which the reaction will proceed at equilibrium.
for more questions on equilibrium
https://brainly.com/question/3159758
#SPJ8
. How many moles of BaSO4 could be obtained from 0.75moles of BaCl2 via the precipitation reaction:
BaCl2(aq) + Na2S04(aq) --> 2NaCl(aq) + BaSO4(s) ?
Answers
Answer:
0.75 moles of BaSO4
Explanation:
Based on the balanced equation:
BaCl2(aq) + Na2S04(aq) --> 2NaCl(aq) + BaSO4(s)
1 mole of BaSO4 is obtained from 1 mole of BaCl2 when Na2SO4 is in excess.
That means if you add 0.75 moles of BaCl2, the moles of BaSO4 that could be obtained are the same:
0.75 moles of BaSO4
Discuss: Mass, Volume, and Density
1. Write a brief summary of the main steps you took in the procedure and what the purpose of each step was. Be sure to include a description of any adjustments you may have made to the procedure and why you made them.
Answers
A substance's mass is a measure of its amount of matter. Volume is a unit used to describe how much space an object occupies. A volume's density tells us how much matter there is in that space.
What are the benefits of mass, volume, and density?Fundamental physical measurements of a thing, such as its mass, volume, and density, can provide important clues about its make-up and condition.
What makes density significant to us?The amount of volume or space that makes up an object or substance is measured by its density. How does density affect each and every part of our existence, then? Gold mining, blood separation, strawberry DNA extraction, and even stacked buildings all depend on density (like the one you see here).
To know more about average visit:-
brainly.com/question/16180989
#SPJ1
Which elements are most likely to react with one another?
K and I
Cs and Br
Na and Br
Cs and I
Answers
I need answer key to edmentum guided notes for Chemistry
Answers
Answer:
I have some answer keys to it. Comment your snapcht and I will send it to you! :)
Explanation:
A certain reaction with an activation energy of 185 kJ/mol was run at 505 K and again at 525 K . What is the ratio of f at the higher temperature to f at the lower temperature
Answers
Answer:
The ratio of f at the higher temperature to f at the lower temperature is 5.356
Explanation:
Given;
activation energy, Ea = 185 kJ/mol = 185,000 J/mol
final temperature, T₂ = 525 K
initial temperature, T₁ = 505 k
Apply Arrhenius equation;
\(Log(\frac{f_2}{f_1} ) = \frac{E_a}{2.303 \times R} [\frac{1}{T_1} -\frac{1}{T_2} ]\)
Where;
\(\frac{f_2}{f_1}\) is the ratio of f at the higher temperature to f at the lower temperature
R is gas constant = 8.314 J/mole.K
\(Log(\frac{f_2}{f_1} ) = \frac{E_a}{2.303 \times R} [\frac{1}{T_1} -\frac{1}{T_2} ]\\\\Log(\frac{f_2}{f_1} ) = \frac{185,000}{2.303 \times 8.314} [\frac{1}{505} -\frac{1}{525} ]\\\\Log(\frac{f_2}{f_1} ) = 0.7289\\\\\frac{f_2}{f_1} = 10^{0.7289}\\\\\frac{f_2}{f_1} = 5.356\)
Therefore, the ratio of f at the higher temperature to f at the lower temperature is 5.356
A researcher observes a reaction and gathers the data in the table below. Observations Mass decreased after reaction Energy is released during reaction New substance is formed Which piece of evidence best identifies they type of reaction as nuclear or chemical? 1. Chemical, because energy is released during the reaction. 2.Nuclear, because energy is released during the reaction. 3.Nuclear, because the mass decreased after the reaction. 4.Chemical, because a new substance is formed.
Answers
The piece of evidence that best identifies the type of reaction as nuclear or chemical is: Chemical, because a new substance is formed. Option 4
In this scenario, the observation that a new substance is formed is a key characteristic of a chemical reaction. Chemical reactions involve the rearrangement of atoms to form different substances with distinct properties. The formation of a new substance indicates a chemical change has occurred.
The other pieces of evidence listed do not necessarily point to a nuclear reaction:
Chemical, because energy is released during the reaction: Energy can be released in both nuclear and chemical reactions, so this observation alone is not sufficient to determine the type of reaction.
Nuclear, because energy is released during the reaction: While energy can be released in nuclear reactions, it is not exclusive to them. Chemical reactions can also release energy, such as in exothermic reactions.
Nuclear, because the mass decreased after the reaction: This observation suggests a change in mass, which could be indicative of a nuclear reaction. However, it is important to consider that chemical reactions can also involve changes in mass, such as the formation of gases or dissolution of a solid.
Overall, the most conclusive evidence to identify the type of reaction is the formation of a new substance, which aligns with a chemical reaction.
Option 4
For more such questions on Chemical visit:
https://brainly.com/question/29886197
#SPJ8