Answers
Answer:
Gene - determines the trait (phenotype)
Related Questions
which transition state is more stable and why? select an answer and submit. for keyboard navigation, use the up/down arrow keys to select an answer. a the transition state that produces the markovnikov product is more stable because a partial positive charge is forming on tertiary carbon your answer b the transition state that produces the markovnikov product is more stable because a partial positive charge is forming on primary carbon c the transition state that produces the anti-markovnikov product is more stable because a partial positive charge is forming on tertiary carbon d the transition state that produces the anti-markovnikov product is more stable because a partial positive charge is forming on primary carbon
Answers
Answer:
The closer the transition state to the product, the more stable it is. It is because this transition state is closest to the final form of the product, which is usually stable
write a balanced equation for the formation of 1 mol of each of the following: liquid methanol (ch3oh) to produce co2 and h2o
Answers
The balanced equation for the formation of 1 mol of liquid methanol (CH₃OH) to produce CO₂ and H₂O is CH₃OH + O2 → CO₂ + 4 H₂O This means that for every mole of methanol that react, 1 mole of carbon dioxide and 2 moles of water are produced.
To write a balanced equation for the formation of 1 mol of liquid methanol (CH₃OH) to produce CO2 and H2O, follow these steps:
1. Write down the reactants and products: CH₃OH (reactant) → CO₂ (product) + H₂O (product)
2. Balance the equation by adjusting the coefficients of the reactants and products.
The balanced equation for the formation of 1 mol of liquid methanol to produce CO₂ and H₂O is:
CH₃OH (l) → CO₂ (g) + 2 H₂O (l)
Learn more about liquid methanol at https://brainly.com/question/13992085
#SPJ11
what are plasmas properties?
Answers
Answer:Plasma is highest energy state of matter.It consists of electrons,protons and neutral particles.
Explanation:(1) Plasma has a very high electrical conductivity .
(2) The motion of electrons and ions in plasma produces it's own electric and magnetic field
(3)It is readily influenced by electric and magnetic fields .
(4)It produces it's on electromagnetic radiations.
What is the smallest unit of life that conducts all life functions?
A. cell
B. organelle
C. tissue
D. organ
Answers
Answer:
a: cells
Explanation:
just did it. your welcome have a nice day
answer :- The smallest unit of life that conducts all life function are cell
Who was the earliest advocate for a uniform measuring system?
Answers
Answer:
James Clerk Maxwell
Explainlation
In the middle of the 19th century, James Clerk Maxwell put forward the concept of a coherent system where a small number of units of measure were defined as base units, and all other units of measure, called derived units, were defined in terms of the base units. Maxwell proposed three base units: length, mass and time
Calculate the molar mass of Na (CI03)
Answers
Molar mass of Na is 23 u
What is molar mass ?
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles.[1] The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.
To know more about molar mass visit
https://brainly.com/question/837939
#SPJ1
4. Anh measured the temperature of a pond near his house. Before he left for school, the water in the
pond was 18°C. When he came home from school, the temperature of the pond was higher than it
was in the morning. What happened to the water molecules while he was at school?
Answers
The water molecules must have gained more thermal energy while Anh was away from the house.
An increase in the temperature of a body means that the body has gained more energy in the form of heat, also known as thermal energy.
The thermal energy represents the characteristic of a body responsible for its temperature.
An increase in the temperature of the water molecules increases their kinetic energy and, thus, makes the water molecules move around faster than before.
More on thermal energy can be found here: https://brainly.com/question/11278589?referrer=searchResults
What are some indicators of rusting? Check all that apply. production of light color change formation of a solid formation of a gas
Answers
Answer:
Some indicators of rusting include:
a. color change
b. formation of as solid
Explanation:
Rusting is a redox ( a reaction in which reduction and oxidation occur concurrently) reaction which occcur when metallic iron is exposed to air and water or water vapor reacts with the oxygen and water to form a hydrated mixture of oxides of the metal.
The shiny metallic iron becomes oxidized to Fe²⁺ and Fe³⁺ ions while neutral oxygen gas molecules are reduced to O²⁻ ions. The shiny metallic iron also loses its lustrous appearance as the process proceeds and is covered by a layer of rust.
The equation for reaction is given below:
4Fe(s) + 30₂(g) + xH₂O(l) -----> 2Fe₂O₃. xH₂O(s)
The product of the reaction above is known as rust and is reddish-brown solid.
Rusting causes iron to become flaky and weak as rust is very light and porous, reducing the strength and appearance of iron which are desirable properties of iron when used in construction. The rusting of iron can lead to damage to automobiles, railings, bridges, and several other iron structures.
Answer:
color change and formation of a solid.
Explanation:
the answer that comes after is chemical also hope this helps!
Where is groundwater stored?
A. in lakes and rivers
B. in oceans
C. in ice caps at the poles
D. in cracks and pores beneath Earth's surface
Answers
Answer:
d
Explanation:
Answer:
Answer is D.
in the cracks and pores beneath Earth's surface
Explanation:
hope this helps!
Pure substances are homogeneous substances made of identical particles called
Answers
Answer:
a pure substance
Explanation:
i hope i help if i didnt im srry:(
What sugar provides energy for all cells of the body?
Help me plz
Answers
In one to two sentences, describe an experiment that would show that intramolecular forces (attractions between atoms within molecules) are stronger than intermolecular forces (attractions between molecules)
Answers
In order to demonstrate that intramolecular forces are stronger than intermolecular forces, a block of ice will be heated in a sealed container until it turns into steam.
Why do intramolecular forces outweigh intermolecular forces?
Because the forces holding together compounds are stronger than the forces holding together molecules, intramolecular forces are stronger than intermolecular forces.
Intermolecular forces exist between molecules, but intramolecular forces exist between atoms within a molecule. This is the primary distinction between intermolecular and intramolecular forces.
Look for the molecule with the most polarity, the most electronegative atoms, or the most hydrogen bonding groups if the molecules have identical molar weights and similar intermolecular forces. That one will have the overall stronger IMFs.
Learn more about intramolecular forces at:
https://brainly.com/question/26096719
#SPJ1
What is solubility a measure of?
A. The amount of solute that will dissolve
B. The type of solute that will dissolve
C. The rate at which the solute will dissolve
D. The energy needed for the solute to dissolve
Answers
Answer:A
Explanation:
I just took the test
What is this help ASAP
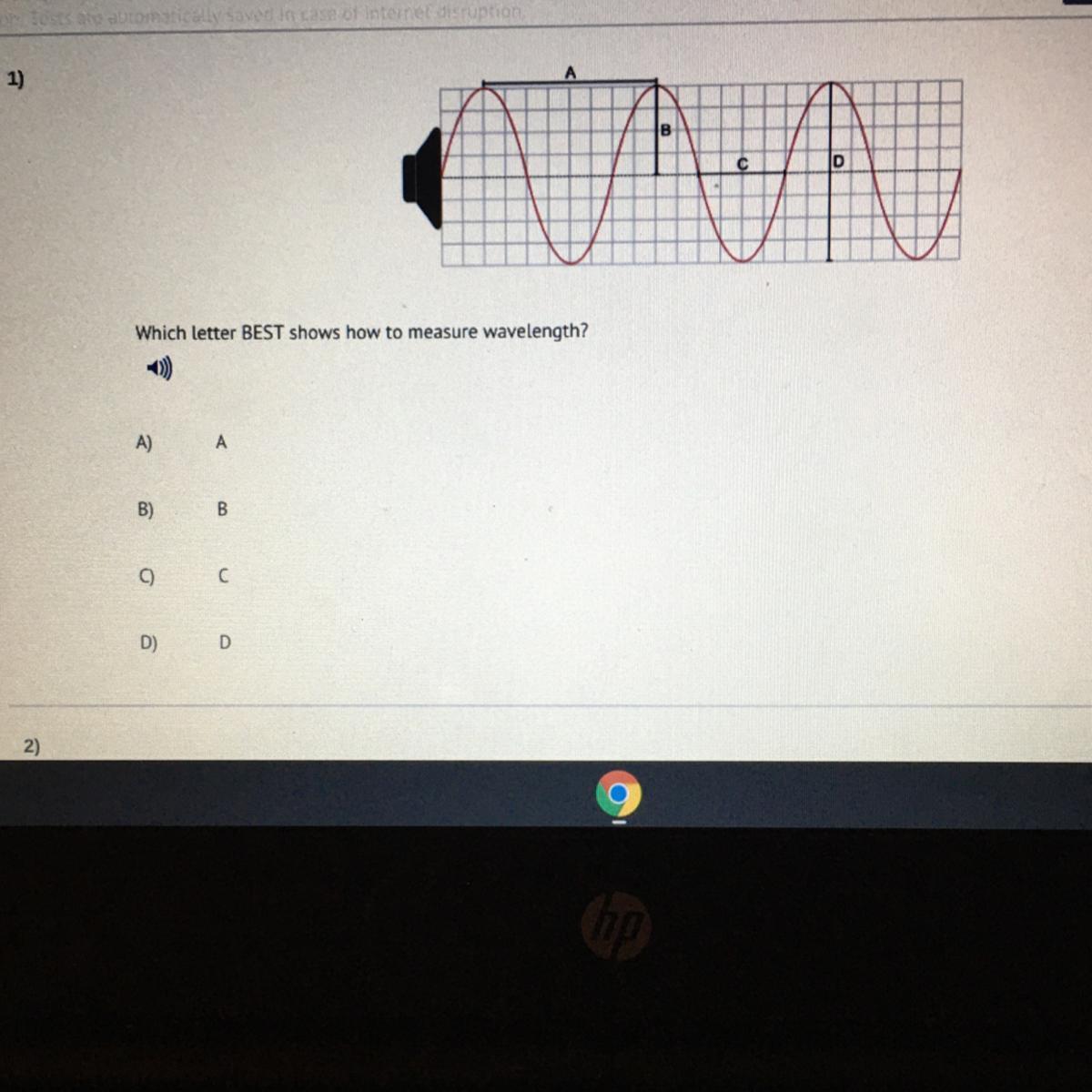
Answers
Answer:
A, im gonna say. Hopefully this helps!
Explanation:
Length is horizontal, and A is horizontal too.
Explain how and why a drop of syrup can spread through the water in a beaker without stirring?
Answers
Answer:
See the answer below
Explanation:
A drop of syrup would spread through water because water molecules interact and dissolve the syrup molecules and the molecules of the syrup randomly diffuse into the water from the region of higher concentration to the region of lower concentration till they become evenly distributed in the water.
Once the molecules become evenly distributed into the water, an equilibrium is established and there is no longer net movement.
If a reaction is performed in 155 g of water with a heat capacity of 4.184 J/g °C and
the initial temperature of a reaction is 19.2°C, what is the final temperature (in units
of °C) if the chemical reaction releases 1420 J of heat?
Answer choices:
21.4
29.2
27.4
34.5
Answers
For this exercise, the formula for calculating heat is needed
\(Q = m × c_{s} × ∆T \)
In this case, we need to fInd the difference in temperature of the water, so
\(∆T = \frac{Q}{m × c_{s}} = \frac{1420 J}{155 g × 4,184 J/g °C} = 2,2 °C\)
Since water accepts heat from the reaction, its temperature increases therefore the final temperature is
\(T_{f} = T_{0} + ∆T = 19,2 °C + 2,2 °C = 21,4 °C\)
The addition of solid Na2SO4 to anaqueous solution in equilibrium with solid BaSO4 willcause
A. no change in [Ba2+] in solution
B. more BaSO4 to dissolve
C. precipitation of more BaSO4
D. an increase in the Ksp of BaSO4
Substance Ksp, 25°C
BaSO4(s) 1.5x 10-9
Answers
The addition of solid Na2SO4 to an aqueous solution in equilibrium with solid BaSO4 will cause precipitation of more BaSO4. The correct answer is option C.
When Na2SO4 is added to the solution, it dissociates into Na+ and SO4^2-. The presence of additional sulfate ions (SO4^2-) in the solution will shift the equilibrium of the BaSO4 dissolution reaction towards the formation of more solid BaSO4.
The chemical equation for the dissolution of BaSO4 is:
BaSO4(s) ⇌ Ba2+(aq) + SO4^2-(aq)
By Le Chatelier's principle, when additional sulfate ions are introduced to the system (by adding Na2SO4), the equilibrium will shift to the left to counteract the increase in sulfate ions. As a result, more solid BaSO4 will be precipitated from the solution.
The Ksp value of BaSO4 indicates that it is sparingly soluble, meaning only a small amount of BaSO4 can dissolve in water. Therefore, when more solid BaSO4 is precipitated, it indicates a decrease in the concentration of Ba2+ ions in the solution.
In summary, the addition of solid Na2SO4 to the equilibrium system will cause precipitation of more BaSO4, leading to a decrease in the concentration of Ba2+ ions in the solution.
Learn more about Le Chatelier's principle here:
https://brainly.com/question/29009512
#SPJ11
where dose everything go plz help!!???!?

Answers
Wedge
Wheel and axel
Inclined plane
Lever
Pulley
Hope that helped!
Wheel go’s to the last one
Inclined plane go’s to the forth one
How is wind energy more environmental friendly than heat energy?
Answers
Answer:
Wind is an emissions-free source of energy
Overall, using wind to produce energy has fewer effects on the environment than many other energy sources. Wind turbines do not release emissions that can pollute the air or water (with rare exceptions), and they do not require water for cooling.
Explanation:
How the calculation of the [OH-], pH and % ionization for 0.619 M ammonia (NH3) NH3 + H2O (liq) rightwards harpoon over leftwards harpoon NH4+ + OH- Kb = 1.8 x 10-5
Answers
Answer:
[OH⁻] = 3.34x10⁻³M; Percent ionization = 0.54%; pH = 11.52
Explanation:
Kb of the reaction:
NH3 + H2O(l) ⇄ NH4+ + OH-
Is:
Kb = 1.8x10⁻⁵ = [NH₄⁺] [OH⁻] / [NH₃]
As all NH₄⁺ and OH⁻ comes from the same source we can write:
[NH₄⁺] = [OH⁻] = X
And as [NH₃] = 0.619M
1.8x10⁻⁵ = [X] [X] / [0.619M]
1.11x10⁻⁵ = X²
3.34x10⁻³ = X = [NH₄⁺] = [OH⁻]
[OH⁻] = 3.34x10⁻³M% ionization:
[NH₄⁺] / [NH₃] * 100 = 3.34x10⁻³M / 0.619M * 100 = 0.54%
pH:
As pOH = -log [OH-]
pOH = 2.48
pH = 14 - pOH
pH = 11.52Draw the Major Organic product of the following reaction. Do NOT use abbreviations such as Ph. Do NOT draw out any hydrogen explicitly. Do NOT include the ionic side product or any other side product such as water, CH3NH2 or CH3NH3+.
Answers
Drawing the major organic product of a reaction requires a thorough understanding of the reaction type, stereochemistry, and functional groups involved. Without more information about the specific reaction, it is impossible to provide a definitive answer.
First, it is important to identify the type of reaction taking place. Organic reactions can be broadly categorized as substitution, elimination, addition, or rearrangement reactions. Each type of reaction has its own characteristic mechanism and product(s).
Second, you should consider the stereochemistry of the reaction. Organic reactions can result in different stereoisomers depending on the orientation of the reactants and the reaction conditions. This can be important for predicting the properties and reactivity of the final product.
Finally, you should consider any functional groups present in the reactants and the possible products. Functional groups are groups of atoms that have characteristic chemical properties and can participate in specific types of reactions. For example, an alcohol (-OH) can undergo an elimination reaction to form an alkene (-CH=CH2), while an alkene can undergo an addition reaction to form an alcohol.
In conclusion, drawing the major organic product of a reaction requires a thorough understanding of the reaction type, stereochemistry, and functional groups involved.
To know more about organic product, refer
https://brainly.com/question/29666831
#SPJ11
In the synthesis of aspirin we react salicylic acid with acetic anhydride. The balanced chemical equation is:2HOOCC6H4OH + C4H6O3 → 2HOOCC6H4O2C2H3 + H2Osalicylic acid acetic acetyl salicylic acid. water anhydride If we mix together 26.3 grams of salicylic acid with 17.7 grams of acetic anhydride in this reaction, we obtain 30.7 grams of aspirin.a. What are the theoretical yields of our experiment? b. What are the percentage yields of our experiment?
Answers
(a). First, we need to identify the limiting reactant. Let's calculate the number of moles of each reactant:
For salicylic acid, we have that its molar mass is 138.1 g/mol (you can calculate it using the periodic table and doing the algebraic sum):
\(26.3\text{ g s. acid}\cdot\frac{1\text{ mol s. acid}}{138.1\text{ g s. acid}}=0.19\text{ mol s. acid.}\)And now, let's see the number of moles of 17.7 g of acetic anhydride, where its molar mass is 102.1 g/mol:
\(17.7\text{ g a. anhydride}\cdot\frac{1\text{ mol a. anhydride}}{102.1\text{ g a. anhydride}}=0.17\text{ mol a. anhydride.}\)Now, you can realize that in the reaction 2 moles of salicylic acid react with 1 mol of acetic anhydride. Let's see how many moles of acetic anhydride requires to react with 0.19 moles of salicylic acid:
\(0.19\text{ mol s. acid}\cdot\frac{1\text{ mol a. anhydride}}{\text{2 mol s. acid}}=0.095\text{ mol a. anhydride.}\)You can realize that we obtained less number of moles for acetic anhydride, meaning that the acetic anhydride is in excess and the salicylic acid is the limiting reactant, so we're going to work with this compound to find how many grams of aspirin is produced:
In the chemical equation, 2 moles of salicylic acid reacted produces 2 moles of aspirin, so the mole ratio between these two is 1:1 which is telling us that 0.19 moles of salicylic acid are producing 0.19 moles of aspirin. With this value, we can calculate the mass of aspirin as our theoretical yield. The molar mass of aspirin is 180.2 g/mol:
\(\text{0}.19\text{ mol aspirin}\cdot\frac{180.2\text{ g aspirin}}{1\text{ mol aspirin}}=34.24\text{ g aspirin.}\)Remember that the theoretical yield is the amount that we expect to get in the reaction, in this case, is 34.24 grams of aspirin.
(b). Let's see the formula for percent yield:
\(\text{percent yield=}\frac{actual\text{ amount of product}}{\text{theoretical }yield}\cdot100,\)where the actual amount of product is what we obtained from the reaction, in this case, 30.7 grams of aspirin and the theoretical yield that we've already found (34.24 g). Replacing these values, we're going to have:
\(\begin{gathered} \text{percent yield=}\frac{30.7\text{ g aspirin}}{34.24\text{ g aspirin}}\cdot100, \\ \text{percent yield=89.66}\%. \end{gathered}\)The percentage yield of the experiment is 89.66%.
Aluminum hydroxide, a popular antacid, is made when aluminum oxide is reacted with water. How many moles of aluminum oxide will be needed to produce .64 moles of aluminum
hydroxide, if there is plenty of water?
Answers
The balanced chemical equation for the reaction between aluminum oxide and water to form aluminum hydroxide is:
Al2O3 + 3 H2O → 2 Al(OH)3
How many moles of aluminum oxide will be needed to produce .64 moles of aluminum hydroxide, if there is plenty of water?We can see that for every 1 mole of Al(OH)3 produced, we need 1/2 mole of Al2O3:
1 mole Al2O3 produces 2 moles Al(OH)3
1/2 mole Al2O3 produces 1 mole Al(OH)3
So, if we need 0.64 moles of Al(OH)3, we can calculate the amount of Al2O3 needed as:
0.64 moles Al(OH)3 x (1/2) moles Al2O3 per 1 mole Al(OH)3 = 0.32 moles Al2O3
Therefore, we need 0.32 moles of aluminum oxide to produce 0.64 moles of aluminum hydroxide, assuming there is plenty of water.
Learn more about Aluminum hydroxide from
https://brainly.com/question/30067644
SPJ1
50 pts! Please help, much appreciated (:
2. Consider the table of boiling points and structural isomers. Note that butane has two possible isomers but that decane has 75 possible isomers. Why does the number of possible isomers go up with an increasing number of carbon atoms?
Answers
Answer:
because the number of constitutional confirmation , and geometric isomers goes up with each carbon atom added there are many more possible configurations and connectivities possible with decane , a 10 carbon chain , than with butane, a 4 carbon chain
"The number of possible isomers go up with an increasing number of carbon atoms because as the number of carbon atoms increases the tendency to form branching with other carbon atom increases as well."
What is isomer?
Isomers are molecules or polyatomic ions that have the same molecular formulas that is, the identical number of atoms of each element but different atomic configurations in space. Isomerism refers to the existence or potential of isomers. Isomers don't have to have the same chemical or physical properties.
To know more about isomer click here.
https://brainly.com/question/23248623.
#SPJ2
54 g of water was produced when a sample of methane was burned in an excess of air. What mass of methane was burned assuming complete combustion?.
Answers
Assuming complete combustion, the stated statement indicates that 24 g of methane were consumed.
What happens during combustion?In the chemical process of burning, an item swiftly combines with oxygen to produce heat. The initial substance and the supply of oxygen are referred to as fuel and oxidizer, respectively. Despite typically being a liquid, fuel for airplane propulsion can also occasionally be a solid, liquid, or gas.
Briefing:
CH4 + 2O₂——————-> CO₂ + 2H₂O
1 mole of methane, or 16 g, will yield =2 x 18 = 36 g of water.
Thus, = 16/36 x 54 = 24 g of methane will be converted into 54 g of water.
To know more about combustion visit:-
https://brainly.com/question/15117038
#SPJ4
what contribution to atomic theory resulted from albert einstein’s work?

Answers
Answer: A new model of the atom that described electrons as being in a cloud
Explanation:
Answer:
A new model of the atom that described electrons as being in a cloud
Explanation:
AP3X
Calculate the unit cell edge length for an 81wt%Fe−19wt% V alloy. All of the vanadium is in solid solution, and, at room temperature the crystal structure for this alloy is BCC. Show all steps. What is the effect of increasing the temperature in this problem? (80 pts)
Answers
The temperature of the crystal is increased, the vibrations of the atoms will become greater, the atoms will have more energy and will move further from their equilibrium position
Given that the alloy is an 81 wt% Fe−19 wt% V alloy, and all vanadium is in solid solution. At room temperature, the crystal structure for this alloy is BCC.
We have to find the unit cell edge length, a and the effect of increasing the temperature.
To calculate the unit cell edge length for an 81 wt% Fe−19 wt% V alloy, we will use the formula;
For BCC, the number of atoms per unit cell (Z) = 2a^3/Z^3Where Z is the coordination number for a BCC lattice.
For BCC, Z= 8 (number of atoms in a unit cell).We know that the atomic weight of Fe and V is 55.85 g/mol and 50.94 g/mol respectively.
Atomic weight of the given alloy = 81 × 55.85 + 19 × 50.94 = 2967.74Atomic radius of Fe = 0.126 nm
Atomic radius of V = 0.134 nm
Now, Unit cell edge length a = 4/√3 × r
Where r = (rFe + rV) /2 = (0.126 + 0.134) / 2 = 0.130 nm
Hence a = 0.287 nm
At room temperature, the crystal structure for this alloy is BCC.
The effect of increasing temperature on this alloy is that it will expand. The lattice parameter will increase and the unit cell edge length will also increase.
When the temperature of the crystal is increased, the vibrations of the atoms will become greater, the atoms will have more energy and will move further from their equilibrium position. This increased movement will cause the lattice to expand, causing the unit cell edge length to increase.
Learn more about equilibrium from the given link
https://brainly.com/question/517289
#SPJ11
Define physical and chemical properties, provide three examples of each, discuss their reversibility, and explain the fundamental differences between them.
Answers
Answer:
Physical properties are defined as the properties which can be observed without changing its chemical composition.
For example: color, volume, and molecular weight.
Chemical properties are defined as the properties which can be observed only after changing chemical identity of the substance.
For example: reactivity, toxicity, and flammability.
The fundamental differences between physical and chemical properties are as follows:
Chemical properties are related to chemical bonds of the substance while physical properties are not.
In chemical properties, chemical identity of substance changes while physical properties do not have any change.
Chemical properties predict the reaction of substance while physical properties only describe the appearance of the substance.
Explanation:
Answer:
A measurable property that can be observed typically and explains any matter or organism's appearance and physical behavior is called a physical property. Examples of physical properties are appearance, boiling point, freezing point, melting point, colors, odor, mass, density, solubility. Properties that are only observed during a reaction or change in the composition of a matter is called a chemical property. These properties describe the internal part of anything and explain how it behaves when reacted with other chemical substances. Examples are enthalpy, entropy, reactivity, flammability, oxidation state, acidity, basicity. Some physical properties are reversible or sometimes irreversible. Like the change of states of matter is reversible, and the growth of an organism is irreversible. The same thing with the chemical properties. There are reversible chemical reactions(formation of ammonium chloride) and irreversible chemical reactions. (burning of a chemical substance). After knowing all about these properties, the most fundamental difference is that the physical properties can be measured without any chemical change in them. But a chemical property is measurable when the substance goes through a chemical reaction.
Explanation:
Please write an explanation if you find the answer, I don't get it.
A flask contains 21.8 g of chlorine gas and 47.8 g of sulfur dioxide gas. What is the mole fraction of the sulfur dioxide?
Round your answer to 3 decimal spaces, even if sig figs are not conserved.
Answers
The mole fraction of the sulfur dioxide gas present in a falsk which contains 21.8 g of chlorine gas and 47.8 g of sulfur dioxide gas is 0.708.
How do we calculate mole fraction?Mole fraction of any substance will be calculated by dividing the moles of desired substance by the total moles of the species present in that sample.
Moles can be calculated as:
n = W/M, where
W = given mass
M = molar mass
Moles of 21.8g of chlorine gas = 21.8g / 71g/mol = 0.307mol
Moles of 47.8g of sulphur dioxide gas = 47.8g / 64g/mol = 0.746mol
Mole fraction of sulphur dioxide gas = 0.746 / 0.746+0.307 = 0.708
Hence required mole fraction of sulphur dioxide gas is 0.708.
To know more about mole fraction, visit the below link:
https://brainly.com/question/1601411
#SPJ1
Determine the freezing point depression of 2 kg of water when 2 mol salt is added to it. The kf of water is 1.86 degrees C/M. Show all your work.
Answers
Answer: The freezing point depression is \(1.86^0C\)
Explanation:
Depression in freezing point is given by:
\(\Delta T_f=K_f\times m\)
\(\Delta T_f\) = Depression in freezing point
\(K_f\) = freezing point constant = \(1.86^0C/m\)
m= molality = \(\frac{\text {moles of solute}}{\text {mass of solvent in kg}}=\frac{2mol}{2kg}=1m\)
\(\Delta T_f=1.86mol/kg^0C\times 1m\)
\(\Delta T_f=1.86^0C\)
Thus freezing point depression is \(1.86^0C\)
