Can a bar graph be turned into a circle graph
Answers
Answer: DUHHHHH!
Explanation:
Related Questions
13. If a chemist has 12.3 moles of N H 03, what is the mass of the sample?
Answers
Answer:
209.4831 g
Explanation:
number of moles × molar mass = mass of substance in g
12.3 × ( 14.0067 + 1.00484 × 3 ) = 209.4831 g
Maria’s class has learned how to drop an egg without breaking it. After 0.2 s, the kinetic energy of the falling egg is 0.5 J. If its mechanical energy is 2.3 J, what is its potential energy?
Answers
The energy an object has during its motion is known as the kinetic energy and the energy stored in an object is called the potential energy. The potential energy of the egg drop is 1.8 J.
What is mechanical energy?The sum of potential energy and kinetic energy of an object is known as the mechanical energy. It describes the energy of an object due to its motion or position or both.
The equation of mechanical energy is:
Mechanical energy = Potential energy + Kinetic energy
Potential energy = Mechanical energy - Kinetic energy
2.3 J - 0.5 J = 1.8 J
Thus the potential energy of the egg drop is 1.8 J.
To know more about potential energy, visit;
https://brainly.com/question/22764
#SPJ1
Which of the following statements is true about most naturally occurring gases? (Hint: consider the air in your front yard as an example)
Select one:
a.
They are compounds.
b.
They are ions.
c.
They are mixtures.
d.
They are elements.
Answers
Most naturally occurring gases are a mixture. This statement is true about most naturally occurring gases.Gases are one of the four fundamental states of matter (solid, liquid, gas, and plasma). So correct answer is C
They are distinguished from other states by their ability to conform to the form of the container in which they are stored (assuming that the container is not entirely sealed). Gases are made up of tiny, discrete molecules that are spread out throughout a large volume, and these molecules can be subjected to an external force such as heat or pressure, which will cause the gas to compress or expand. These molecules do not interact with one another in the same way that liquids or solids do, as they are free to move and do not have a definite shape or volume.
To know more about Gases visit:
brainly.com/question/28307007
#SPJ11
What are the types of ions found in an ionic bond? (Check all that apply) * 2 points
cations
anions
prions
quarks
Answers
The right options is a cation and an anion !
even though b contains three ester groups, a single dieckmann product results when b is treated with naoch3 in ch3oh, followed by h3oPart 1: Why is only one product formed from B?
Answers
Because the two ester groups that react are found in the same molecule, the reaction is intramolecular. As a result, one cyclic β-ketoester product is produced.
What Dieckmann reaction?The Dieckmann reaction is the name for intramolecular Claisen condensation in dibasic acid esters. Cycle 13-ketone derivatives are always the end products. The condensing bases could be potassium t-butoxide, sodium, sodium ethoxide, sodium hydride, etc.
The reason only one product is formed from B is because the reaction conditions promote intramolecular cyclization via the Dieckmann condensation reaction. This reaction involves the formation of a cyclic β-ketoester by the condensation of two ester groups within the same molecule. In the case of B, the presence of three ester groups might suggest the formation of three different cyclic products. However, the reaction conditions used in this case, i.e., treatment with sodium methoxide in methanol followed by acid workup, promote selective formation of the most stable cyclic β-ketoester product, which is the only observed product.
The reaction occurs in the following steps:
1. Deprotonation of one of the ester groups by sodium methoxide to form an enolate intermediate.
2. Nucleophilic attack by the enolate on the adjacent ester group, resulting in cyclization and formation of a five-membered ring.
3. Protonation of the intermediate by water in the acidic workup step to form the final product.
The reaction is intramolecular because the two ester groups that react are present in the same molecule. This leads to the formation of a single cyclic β-ketoester product.
Learn more about Dieckmann Reaction on:
https://brainly.com/question/30217449
#SPJ11
For the reaction: 2HgO(s) 2Hg() + O2(g), how many grams of O2 are produced from 10 moles of mercury(II)oxide?
Answers
For the reaction: 2HgO(s) → 2Hg(l) + O₂(g), 160 grams of O₂ are produced from 10 moles of mercury(II) oxide. To find the grams of O₂ produced from 10 moles of mercury(II) oxide, follow these steps below.
Write down the balanced chemical equation:
2HgO(s) → 2Hg(l) + O₂(g)
Determine the stoichiometric ratio between HgO and O₂:
According to the balanced equation, 2 moles of HgO produce 1 mole of O₂.
Calculate the moles of O₂ produced:
Since you start with 10 moles of HgO, the moles of O2 produced can be found using the stoichiometric ratio:
(10 moles HgO) × (1 mole O₂ / 2 moles HgO) = 5 moles O₂
Convert moles of O₂ to grams:
The molar mass of O₂ is 32 g/mol (16 g/mol for each oxygen atom). To convert the moles of O₂ to grams, multiply the moles by the molar mass:
(5 moles O₂) × (32 g/mol) = 160 grams
Learn more about the mass: https://brainly.com/question/19694949
#SPJ11
) cobalt is an essential component of what common product (one that is found in cell phones and many other products)?
Answers
Cobalt is an essential component of rechargeable batteries. It is found in cell phones and many other products.
Cobalt is a chemical element with the symbol Co and atomic number 27. It is a hard, grey metal that is used in the production of rechargeable batteries, which are a common component of cell phones and many other products.
Besides rechargeable batteries, cobalt is also used in many other products, including aircraft engines, gas turbines, and magnetic alloys. It is also used in some forms of cancer treatment as a radioactive isotope and is an essential nutrient for some bacteria and animals.
Cobalt is also used in the production of pigments and dyes and as a catalyst in some chemical reactions.
Learn more about cobalt :
brainly.com/question/19863670
#SPJ11
Which food provides the most magnesium per serving?
a. canned tuna
b. halibut
c. eggs
d. cheddar cheese
e. ground beef
Answers
According to the statement Halibut food provides the most magnesium per serving.
What advantages does magnesium possess?Along with many other essential tasks, manganese helps the body's muscles, neurons, and energy production. Usually, neither symptom occurs when serum sodium levels are low. Conversely, consistently low levels can increase your risk of coronary disease, prediabetes, hypertension, osteoporosis, especially high blood pressure.
What happens when magnesium is consumed?Mg is a nutrient that the body needs to be healthy. Magnesium is necessary for many internal processes, including the production of DNA, calcium, and proteins as well as the dilation of blood vessels, sugar levels, and the health of muscles and neurons.
To know more about Magnesium visit:
https://brainly.com/question/1533548
#SPJ4
what is the chemical formula for Mg2+ and I1- ( magnesium and iodine)
Answers
M
g
2
+
+
2
I
−
→
M
g
I
2
Answer:MgI2
Explanation:
In a solid, atoms and their bonds are always vibrating. As temperature increases, the vibrations _____, leading to melting. Pressure has the opposite effect, compressing the solid, making it more resistant to melting.
Answers
increasing temperature enhances the vibrational energy of atoms, promoting melting, while increasing pressure compresses the solid, strengthening intermolecular forces and making it more resistant to melting.
In a solid, atoms and their bonds are always vibrating. As temperature increases, the vibrations of the atoms become more energetic and intense. This increased thermal energy disrupts the ordered arrangement of the atoms, causing them to vibrate more vigorously. Eventually, the vibrational energy overcomes the intermolecular forces holding the solid together, leading to a phase transition from solid to liquid, which is known as melting.
On the other hand, pressure has the opposite effect on a solid. When pressure is applied to a solid, it compresses the atoms and reduces the space between them. This compression increases the strength of the intermolecular forces, making it more difficult for the atoms to move and break free from their fixed positions. Consequently, the solid becomes more resistant to melting under high pressure.
To know more about Atom related question visit:
https://brainly.com/question/1566330
#SPJ11
How many moles of Ba3(PO4)2 can be made from 2. 70 moles of Na3PO4?
Answers
2.25 moles of Ba3(PO4)2 can be made from 2.70 moles of Na3PO4. The balanced chemical equation for the reaction between Na3PO4 and BaCl2 is as follows: 2Na3PO4 + 3BaCl2 → Ba3(PO4)2 + 6NaCl
From the equation, we can see that 2 moles of Na3PO4 are needed to produce one mole of Ba3(PO4)2. Therefore, we can use the mole ratio from the balanced chemical equation to determine the number of moles of Ba3(PO4)2 that can be made from 2.70 moles of Na3PO4:
2Na3PO4 → 1Ba3(PO4)2
2.70 moles Na3PO4 x (1 mole Ba3(PO4)2 / 2 moles Na3PO4) = 1.35 moles Ba3(PO4)2
However, we must note that the stoichiometric ratio involves two moles of Na3PO4, and therefore, we need to account for the remaining moles of Na3PO4 once the reaction has occurred. The limiting reactant will be the reactant that is completely consumed and determines the amount of the product that can be formed.
When 1.35 moles of Ba3(PO4)2 are produced, the number of moles of Na3PO4 remaining will be:
2.70 moles Na3PO4 - (1.35 moles Ba3(PO4)2 x 2 moles Na3PO4 / 1 mole Ba3(PO4)2) = 0 moles Na3PO4
Therefore, the maximum amount of Ba3(PO4)2 that can be produced is 1.35 moles. However, since we cannot have a fraction of a mole of a substance, we must round down to the nearest whole number of moles. Thus, we can conclude that 2.25 moles of Ba3(PO4)2 can be made from 2.70 moles of Na3PO4.
In summary, 2.25 moles of Ba3(PO4)2 can be produced from 2.70 moles of Na3PO4. The calculation involved using the mole ratio from the balanced chemical equation to determine the number of moles of Ba3(PO4)2, then accounting for the limiting reactant to obtain the final answer. The calculation is essential in determining the proper amounts of reactants that will result in the production of a certain amount of product from a known reactant.
To know more about balanced chemical equation, visit:
https://brainly.com/question/23877810
#SPJ11
What effect does fermented sugar have in beer brewing
Answers
Answer:
If the sugar concentration level of the must becomes too high at any given point--either at the beginning or during the fermentation--it starts to have an inhibiting effect on the yeast's ability to produce alcohol. In other words, the higher sugar concentration starts to act as a preservative effecting the fermentation in a negative way.
Explanation:
Fermentation is the process by which yeast converts the glucose in the wort to ethyl alcohol and carbon dioxide gas -- giving the beer both its alcohol content and its carbonation.
Discuss briefly the colligative property of C12H22O11 and CO (NH2)2.
Answers
Answer: The quantity of solute particles in a solution determines its colloidal qualities, not its chemical composition. Boiling point elevation and freezing point depression are two widely researched colligative features.
Explanation: Sugar is a non-electrolyte solute that does not separate into ions in water (C12H22O11). The amount of dissolved sucrose molecules affect the colloidal characteristics of the substance.
Urea, or CO (NH2)2, is a solute that quickly dissociates into ions in water. Compared to non-electrolytes like sucrose, it adds more particles to the solution, which has a greater impact on the colligative qualities.
A major component of gasoline is octane. When liquid octane is burned in air it reacts with oxygen gas to produce carbon dioxide gas and water vapor. Calculate the moles of octane needed to produce 2.40 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.
Answers
Answer:
0.267 mol
Explanation:
The unbalanced equation for the reaction that takes place is:
C₈H₁₈ + O₂ → H₂O + CO₂Once we balance it we're left with:
2C₈H₁₈ + 25O₂ → 18H₂O + 16CO₂Using the stoichiometric ratio of water and octane from the balanced reaction, we can convert mol of water into mol of octane:
2.40 mol H₂O * \(\frac{2molC_8H_{18}}{18molH_2O}\) = 0.267 mol C₈H₁₈Thus 0.267 moles of octane are needed to produce 2.40 mol of water.
Help!
Which of the following can scientists NOT interpret by examining fossils?
A. How earth's environment has changed overtime.
B. How plants and animals have changed overtime.
C. The age of certain layers of rocks. D. How the pull of gravity has changed.

Answers
Complete the table.
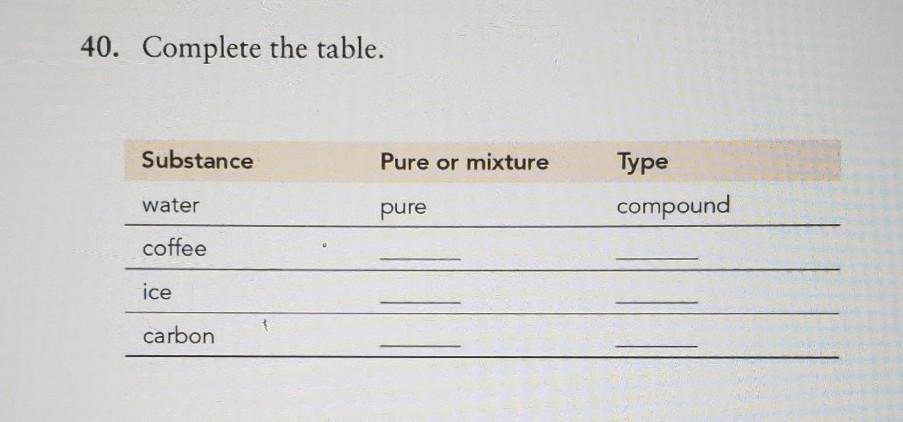
Answers
Explanation:
coffee mixture compound
ice. pure. single
carbon pure. compound
is this reaction feasible?

Answers
Potassium metal reacts with chlorine gas to form solid potassium chloride. Answer the following:
Write a balanced chemical equation (include states of matter)
Classify the type of reaction as combination, decomposition, single replacement, double replacement, or combustion
If you initially started with 78 g of potassium and 71 grams of chlorine then determine the mass of potassium chloride produced.
Answers
The 149.2 grams of potassium chloride would be produced if 78 grams of potassium and 71 grams of chlorine completely reacted.
The balanced chemical equation for the reaction between potassium metal (K) and chlorine gas (Cl₂) to form solid potassium chloride (KCl) is:
2K(s) + Cl₂(g) → 2KCl(s)
This equation indicates that two atoms of potassium react with one molecule of chlorine gas to yield two molecules of potassium chloride.
The type of reaction is a combination reaction, also known as a synthesis reaction. In this type of reaction, two or more substances combine to form a single product.
To determine the mass of potassium chloride produced, we need to calculate the limiting reactant. The molar mass of potassium is approximately 39.1 g/mol, and the molar mass of chlorine is approximately 35.5 g/mol.
First, we convert the given masses of potassium (78 g) and chlorine (71 g) into moles by dividing them by their respective molar masses:
Moles of potassium = 78 g / 39.1 g/mol = 2 mol
Moles of chlorine = 71 g / 35.5 g/mol ≈ 2 mol
Since the reactants have a 1:1 stoichiometric ratio, it can be seen that both potassium and chlorine are present in the same amount. Therefore, the limiting reactant is either potassium or chlorine.
Assuming potassium is the limiting reactant, we can calculate the mass of potassium chloride produced. Since 2 moles of potassium react to form 2 moles of potassium chloride, we can use the molar mass of potassium chloride (74.6 g/mol) to calculate the mass:
Mass of potassium chloride = 2 mol × 74.6 g/mol = 149.2 g
For more such questions on potassium chloride
https://brainly.com/question/24879357
#SPJ11
Calculate the Gibbs energy, entropy, and enthalpy of mixing when 1.00mol C6H14 (hexane) is mixed with 1.00mol C7H16 (heptane) at 298K. Treat the solution as ideal.
Answers
With Hexane of 1.00 ml and 1.00 ml of hectane at 298, the calculated
Gibbs energy = 3.43Entropy = -3.43Enthalpy = 0How to solve for the Gibbs energyC6H14 = C6H14/C6H14+C7H16
1mol/1mol+1mol = 1/2 = 0.5 mol
1+1 x 8.3145J x 298 x 0.5ln0.5 + 0.5ln0.5
= 4955.442 0.6932
= 3435J
convert to KJ
G mix = 3.43
The Gibbs energy is therefore 3.43KJ/mol
The entropy of the solution-(1+1 x 8.3145J x 298 x 0.5ln0.5 + 0.5ln0.5)
S mix = -3.43 KJ/mol
3.43/298 = 11.5
The enthalpy of the solution
3.43-3.43 KJ/mol
H mix = 0
Read more on gibbs solution here: https://brainly.com/question/17310317
how many moles do you have in 37.3 g of Co(CrO4)3
Answers
Answer:
0.0917 mol Co(CrO₄)₃
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:
Step 1: Define
37.3 g Co(CrO₄)₃
Step 2: Identify Conversions
Molar Mass of Co - 58.93 g/mol
Molar Mass of Cr - 52.00 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of Co(CrO₄)₃ - 58.93 + 3(52.00) + 12(16.00) = 406.93 g/mol
Step 3: Convert
\(37.3 \ g \ Co(CrO_4)_3(\frac{1 \ mol \ Co(CrO_4)_3}{406.93 \ g \ Co(CrO_4)_3} )\) = 0.091662 mol Co(CrO₄)₃
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
0.091662 mol Co(CrO₄)₃ ≈ 0.0917 mol Co(CrO₄)₃
Describe the structure of ammonium lauryl sulfate. Refer to the given diagram. Your answer should include the type of bonding, the elements contained, and the size and shape of the molecule. Write a short paragraph.

Answers
Answer:
Answer:
This ammonium laurel sulfates anion consists of a nonpolar hydrocarbon chain and a polar sulfate end group. It means it has a hydrophilic head and hydrophobic tail. There are ammonium ions, sulfate, and fatty acids present.
Lauryl sulfate has lauric acid attach to sulfate ions with carbon-sulfur bond, the fatty acid formed by the covalent bonds between C-C attached to hydrogens. Sulfur also bound to oxygen by covalent bonds. Nitrogen is surrounded by the four hydrogen atoms in the hydrophilic head.
What is the number of collaborative relationships he creates
Each of the carbon atoms in fullerene
Answers
Answer:
answer is 60
Explanation:
Question 8 of 35
Which item rotates in a magnetic field as an electric motor produces kinetic
energy?
A. Permanent magnet
B. Loop of wire
C. Lightbulb
D. Battery
SUBMIT
Answers
You can download answer here
tinyurl.com/wpazsebu
17. saccharin, an artificial sweetener that is 3000 times sweeter than sucrose, is composed of
45.90% carbon, 2.73% hydrogen, 26.23% oxygen, 7.65% nitrogen, and 17.49% sulfur. is the molecular formula of saccharin (a) c14h10o6n2s2, (b) csh,ons, (c) c&h9o2ns, and following orition: com 12.0%
(d) c;h5o3ns?
Answers
Saccharin, an artificial sweetener that is 3000 times sweeter than sucrose, is composed of a) C₁₄H₁₀O₆N₂S₂.
45.90% carbon, 2.73% hydrogen, 26.23% oxygen, 7.65% nitrogen, and 17.49% sulfur. is the molecular formula of saccharin.
To determine the molecular formula of saccharin, we first need to calculate the empirical formula using the given percentages of each element.
Assuming we have 100 grams of saccharin, we have:
Carbon: 45.90 g / 12.01 g/mol = 3.82 mol
Hydrogen: 2.73 g / 1.01 g/mol = 2.70 mol
Oxygen: 26.23 g / 16.00 g/mol = 1.64 mol
Nitrogen: 7.65 g / 14.01 g/mol = 0.55 mol
Sulfur: 17.49 g / 32.07 g/mol = 0.55 mol
We can divide each value by the smallest one, which is 0.55 mol, to get the following ratios:
Carbon: 3.82 / 0.55 = 6.95
Hydrogen: 2.70 / 0.55 = 4.91
Oxygen: 1.64 / 0.55 = 2.98
Nitrogen: 0.55 / 0.55 = 1
Sulfur: 0.55 / 0.55 = 1
The resulting ratios are close to whole numbers, so we can assume the empirical formula to be C₇H₅NO₃S. To find the molecular formula, we need to determine the actual molecular mass of saccharin.
The empirical formula mass of C₇H₅NO₃S is approximately 183 g/mol. The molecular mass of saccharin is known to be around 452 g/mol, so we can calculate the ratio of the molecular mass to the empirical formula mass:
452 g/mol / 183 g/mol = 2.47
This means that the molecular formula is 2.47 times the empirical formula, or:
C₇H₅NO₃S * 2.47 = C₁₇H₁₃N₂O₅S
Therefore, the molecular formula of saccharin is (a) C₁₄H₁₀O₆N₂S₂. The other options (b) CSH,ONS, (c) C&H₉O₂NS, and (d) C;H₅O₃NS are not correct.
To know more about the saccharin refer here :
https://brainly.com/question/31703738#
#SPJ11
An energy level is placed where an ____is most likely to be found in an atom
Answers
Answer: Electron
Explanation:An electron is most likely to be found in an energy level within an atom.
Give the formula for the compound formed between the following ions:
Aluminum and hydroxide
2 pc
Answers
Answer:
Al(OH)3
Explanation:
-Aluminum hydroxide is an antacid. Aluminum hydroxide is used to treat heartburn, upset stomach, sour stomach, or acid indigestion. Aluminum hydroxide is also used to reduce phosphate levels in people with certain kidney conditions. Aluminum hydroxide may also be used for purposes not listed in this medication guide.
-Aluminium hydroxide is an inorganic basic compound used as an intermediary in organic synthesis and as an additive in pharmaceutical and fine chemical industries. Formula and structure: The aluminum hydroxide chemical formula is Al(OH)3 and its molar mass is 78.00 g mol-1.
PLEASE JUST PLEASEEE ANSWER THEM PLSSSS!!! I can’t fail bro plsss

Answers
Explanation:
K = °C + 273
1. -234 + 273 = 39K
2. 84 + 273 = 357K
3. 70 + 273 = 343K
4. -24 + 273 = 249K
5. 134 + 273 = 407K
6. 120 + 273 = 393K
-234 °C = 39K
84 °C = 357K
70 °C = 343K
-24 °C = 249K
134 °C = 407K
120 °C = 393K
Answer:
-234°C = 39K
84°C = 357K
70°C = 343K
-24°C = 249K
134°C = 407K
120°C = 393K
Explanation:
Hope it helps:)
CL1
Gold, one of the most sought-after metals in the world, has a
density of 19. 3 g/cm', a melting point of 1064 °C, and a spe-
cific heat of 0. 129 J/g °C. A gold nugget found in Alaska in
1998 weighed 20. 17 lb. (2. 4. 2. 6, 2. 7. 3. 3. 3. 5)
Gold nuggets, also called
native gold, can be found in
streams and mines.
a. How many significant figures are in the measurement of
the weight of the nugget?
b. Which is the mass of the nugget in kilograms?
c. If the nugget were pure gold, what would its volume be in
cubic centimeters?
d. What is the melting point of gold in degrees Fahrenheit
and kelvins?
e. How many kilocalories are required to raise the tempera-
ture of the nugget from 500. °C to 1064 °C?
f. If the price of gold is $42. 06 per gram, what is the nugget
worth in dollars?
Answers
There are 4 significant figures in the measurement of the weight of the nugget,mass of the nugget in kilograms is 9.15 kg,volume is 474.09 cm³,melting point of gold in degrees Fahrenheit and kelvins is 1064°C and 1948°F.
What are significant figures?Significant figures are used for establishment of a number which is presented in the form of digits. These digits give a meaningful representation to the numbers.
The significant figures are the significant digits which convey the meaning according to the accuracy. These provide precision to the numbers and hence are called as significant numbers.
Volume is found out as, mass/density which 9150/19.3=474.09 cubic centimeters.
Learn more about significant figures,here:
https://brainly.com/question/29153641
#SPJ4
In order to model a chemical change in the laboratory, a student wants to select the appropriate measuring apparatus to record the reaction. Which apparatus could be used to detect that a chemical change occurred with a substance in a laboratory setting? Select ALL that apply.
A) The student could use a hand lens to see if the original substance had been crushed into smaller particles.
B) The student could perform an odor test to detect if a new smell emerges from the interaction of the substance in the lab.
C) The student could test whether gas was released during the interaction of the substance with another substance in the lab.
D) The student could use a spatula to determine if a precipitate/solid formed at the bottom of the beaker containing the substance. Eliminate E) The student could use a balance and a volumetric flask to determine the mass and volume of the substance in order to determine its density.
Answers
Answer:
b,c,d
Explanation:
bc i got it right
The apparatus that could be used to detect that a chemical change occurred with a substance in a laboratory setting are as follows:
The student could perform an odor test to detect if a new smell emerges from the interaction of the substance in the lab.The student could test whether gas was released during the interaction of the substance with another substance in the lab.Thus, the correct options for this question are B and C.
What is a Chemical change?A chemical change may be defined as an alteration in the properties of one material into another. New materials or substances are formed with different properties along with appearance. It results when a substance combines with another to form a new substance.
An odor test and detecting the liberation of gas in the experiment are some of the best ways to reveal whether a chemical change may occur during the reaction or not, while the rest of the options are not as much as practical.
Therefore, the correct options for this question are B and C.
To learn more about Chemical change, refer to the link:
https://brainly.com/question/1222323
#SPJ2
Which two physical properties are used to describe matter?
A Mass and reactivity
B Weight and toxicity
C Volume and pH
D Mass and volume