Calculate the solubility of calcite (caco3) at ph 6.3 and 10.3 assuming a total carbonate of 5 mm. the ksp of calcite is 3.36 x10-9.
Answers
The Ph = 10.3 and S = 8.287 x \(10^{-5}\)
CalculationCaCO3 → Ca^+2 + \(CO_{3} ^{-2}\)
Ksp = [Ca^+2] [ \(CO_{3} ^{-2}\)] = s^2
We need to take into account the hydrolysis of carbonate ion with
K2 = 4.1 x \(10^{-11}\)
H\(w_{3}^{-}\) ⇄ H+ + \(w_{3}^{-2}\)
K2 = \(\frac{[H+][CO_{3}^{-2}] }{[HW_{3}^{-}] }\)
S = \(\sqrt{Ksp}\)
S = \([Ca^{+2}] = [CO_{3}^{-2}] + [HW_{3}^{-}]\)
On putting the values, we will get
\([Ca^{+2}] = [CO_{3}^{-2}] +(1+ \frac{H^{+} }{K_{2} })\)
S^2 = Ksp (1 + \((1+ \frac{H^{+} }{K_{2} })\))
S = \(\sqrt{ Ksp (1+ \frac{H^{+} }{K_{2} })}\)
at Ph = 6.3
[H+] = 5.011 x \(10^{-7}\)
S = \(\sqrt{3.36 * 10^{-9} * (1+\frac{5.011*10^{-11} }{4.8*10^{-11} }) }\)
S = 5.92 x \(10^{-3}\)
at Ph = 10.3
[H+] = 5.011 x \(10^{-11}\)
S = \(\sqrt{3.36 * 10^{-9} * (1+\frac{5.011*10^{-11} }{4.8*10^{-11} }) }\)
S = 8.287 x \(10^{-5}\)
To know more about solubility, visit:
https://brainly.com/question/8591226
#SPJ4
Related Questions
A student places a small stone with a mass of 5 g into a graduated cylinder containing 50 mL of water. With the stone in the graduated cylinder, the water rises to 75 mL. What is the density of the stone? a. 0.07 g/mL b. 0.20 g/mL c. 5.00 g/mL d. 125.00 g/mL
Answers
Answer:
0.20 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
volume = final volume of water - initial volume of water
volume = 75 - 50 = 25 mL
From the question we have
\(density = \frac{5}{25} = \frac{1}{5} = 0.2 \\ \)
We have the final answer as
0.20 g/mLHope this helps you
Amanda dissolves some sugar in water in a beaker. She allows the water to evaporate and notices that solid sugar remains in the bottom of the beaker. What two pieces of evidence can be provided to show that this is a physical change?
Answers
Answer:
evaporation means the state of the water went from liquid to gas which is a physical change. the sugar is still in the beaker which means its chemical properties did not change.
Explanation:
What sorts of additional interactions are taken into account when doing solubility calculations with activities, as opposed to those calculated only using molarities?.
Answers
Addition of anything which increases the ionic interaction of the solution may change the solubility.
What is Solubility?This is defined as the ability of a substance(solute) to form a solution with a solvent such as water.
In the calculation of a solubility activities it is best to take note of any additional substance as it could increase the ionic interactions thereby leading to a change in solubility.
Read more about Solubility here https://brainly.com/question/23946616
The interactions taken into account when doing solubility calculations is that if we add anything which increases the ionic interaction of the solution this may change the solubility of the solute.
What is solubility?Solubility is the degree to which a substance dissolves in a solvent to make a solution.
In this case, the interactions taken into account when doing solubility calculations is that if we add anything which increases the ionic interaction of the solution this may change the solubility of the solute.
Learn more about solubility on:
https://brainly.com/question/23946616
Can u please answer if you know.

Answers
Answer:
plants
Explanation:
they are plant cells, happy Thanksgiving :)
Answer: photosynthetic tissues
Explanation: Chloroplasts are present in the cells of all green tissues of plants and algae. Chloroplasts are also found in photosynthetic tissues that do not appear green, such as the brown blades of giant kelp or the red leaves of certain plants.
An acid is added to water, and a new equilibrium is established. What is the system after the acid is added? A. pH w = 1 x 10-14 B. pH w -14 C. pH > pOH and Kw = 1 x 10-14 D. pH > pOH and Kw > 1 x 10-14
Answers
Answer:
C. pH > pOH; Kw = 1.0 * 10^-14
Explanation:
The ion product of water, Kw = [H+]*[OH-] = 1.0 * 10^-14. It is a constant.
When an acid or base is added to water, its ion product does not change as it a constant. However, the relative concentrations of H+ ions and OH- ions will change depending on whether an acid or base is added to water.
When an acid is added to water, the concentration of H+ ions increases while that of OH- ions decreases, and vice versa.
Therefore, in the above situation where an acid is added to water, pH > pOH; Kw = 1.0 * 10^-14
explain why the other 3 answer options are incorrect.

Answers
Compounds are always uncharged
experiment 1: calculate the combined mass of the two reactants: hydrochloric acid and sodium hydroxide
Answers
The combined mass of hydrochloric acid and sodium hydroxide is determined by adding their individual masses.
When calculating the combined mass of hydrochloric acid and sodium hydroxide, we need to consider the individual masses of these two substances. Hydrochloric acid (HCl) has a molecular formula of HCl and consists of one hydrogen atom (H) and one chlorine atom (Cl). Sodium hydroxide (NaOH), on the other hand, is composed of one sodium atom (Na), one oxygen atom (O), and one hydrogen atom (H). To calculate the combined mass, we add the individual masses of these reactants.
The molar mass of hydrogen (H) is approximately 1 gram/mol, while the molar mass of chlorine (Cl) is approximately 35.5 grams/mol. Sodium (Na) has a molar mass of around 23 grams/mol, oxygen (O) has a molar mass of approximately 16 grams/mol, and hydrogen (H) has a molar mass of around 1 gram/mol.
To determine the combined mass of hydrochloric acid and sodium hydroxide, we multiply the number of atoms of each element by their respective molar masses and sum them up. For example, hydrochloric acid has one hydrogen atom and one chlorine atom, so the total mass would be 1 gram/mol (hydrogen) + 35.5 grams/mol (chlorine). Similarly, sodium hydroxide has one sodium atom, one oxygen atom, and one hydrogen atom, resulting in a combined mass of 23 grams/mol (sodium) + 16 grams/mol (oxygen) + 1 gram/mol (hydrogen).
Learn more about hydrochloric acid
https://brainly.com/question/1451933
#SPJ11
2Al2 (CO3 )3 + 4H3 PO4 ---> AlPO4 + CO2 + H2O
Insert the correct number of atoms from each element from the reactant side of the equation
Al =
C =
O =
H =
P =
Answers
The correct number of atoms from each element from the reactant side of the equation are Al = 4, C = 6, O = 18, H = 36, P = 8.
On the reactant side, how many atoms are there?On the reactant side and the product side, there is one carbon atom each. The same is true for oxygen, although oxygen has two atoms on each side instead of one (remember that the subscript of two in the oxygen molecule means that there are two oxygen atoms bonded together).
Is O2 a product or a reactant?Oxygen and hydrogen gases are both made up of diatomic (two-atom) molecules. The reactants in the process are these molecules.
To know more about element visit:-
brainly.com/question/30858299
#SPJ1
How many liters does a 70. 9 gram sample of Cl2 (g) occupy at STP?
A. 5. 60 L
B. 11. 2 L
C. 22. 4 L
D. 44. 8 L
Answers
70.9-gram sample of \(Cl_{2}\) gas will occupy Opton C. 22.4 liters at STP.
To determine the volume occupied by the sample of \(Cl_{2}\) (g) at STP, we can use the ideal gas law equation, PV = nRT
where P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature.
At STP (Standard Temperature and Pressure), the pressure is 1 atmosphere (atm) and the temperature is 273.15 Kelvin (K).
First, calculate the number of moles of \(Cl_{2}\) (g) using its molar mass. The molar mass \(Cl_{2}\) is 70.9 grams/mol.
Number of moles (n) = mass (m) / molar mass (M)
n = 70.9 g / 70.9 g/mol
n = 1 mol
Now, we can calculate the volume using the ideal gas law:
V = (nRT) / P
V = (1 mol * 0.0821 L·atm/mol·K * 273.15 K) / 1 atm
V ≈ 22.4 L
Therefore, the correct answer is C. 22.4 L.
Know more about STP here:
https://brainly.com/question/13656229
#SPJ8
2.4(a) a sample consisting of 1.00 mol of perfect gas atoms, for which cv,m = –32r, initially at p1 = 1.00 atm and t1 = 300 k, is heated reversibly to 400 k at constant volume. calculate the final pressure, ∆u, q, and w.
Answers
The sample of 1.00 mol of perfect gas atoms, with a molar heat capacity at constant volume (cv,m) of -32R, is heated reversibly from an initial temperature of 300 K to a final temperature of 400 K at constant volume. We need to calculate the final pressure, change in internal energy (∆U), heat (q), and work (w) for this process.
Since the process occurs at constant volume, the work done (w) is zero, as there is no expansion or compression of the gas. The change in internal energy (∆U) can be calculated using the equation:
∆U = q - w
As w is zero, ∆U is equal to q. To find q, we can use the equation:
q = n * cv,m * ∆T
where n is the number of moles of gas and ∆T is the change in temperature.
Given that n = 1.00 mol, cv,m = -32R, and ∆T = 400 K - 300 K = 100 K, we can substitute these values into the equation to find q:
q = (1.00 mol) * (-32R) * (100 K)
The final pressure (P₂) can be calculated using the ideal gas law equation:
P₁V₁ / T₁ = P₂V₂ / T₂
Since the volume (V₁ = V₂) and the gas constant (R) cancel out in this case, we can simplify the equation to:
P₂ = P₁ * (T₂ / T₁)
Substituting the given values, we find:
P₂ = (1.00 atm) * (400 K / 300 K)
By evaluating the above expressions, we can find the final pressure (P₂), change in internal energy (∆U = q), and work (w = 0) for the reversible heating process.
Learn more about pressure here: https://brainly.com/question/30668745
#SPJ11
Identify whether each element is a halogen, a noble gas, or nonmetal only.
Astatine (At):
Nitrogen (N):
Krypton (Kr):
Chlorine (Cl):
Sulfur (S):
Answers
Answer:
Astatine: Halogen
Nitrogen: Non-Metal
Krypton: Non-Metal, Noble Gas
Chlorine: Non-Metal
Sulfur: Non-metal
Explanation:
Gas X has the molecular formula C5Hx. A mass of 1.44g X occupies a volume of 0.32 dm*3 at 2 atm and 400K. Find the value of x. [H=1; C=12; R =0.08 atm.dm3 K*-1.mol*-1]
Answers
The value of x in the gas C₅Hx is 12.
Calculation:-
PV = nRT
n = PV/RT
= 2 × 0.32 / 0.08 × 400
= 0.64 / 32
= 0.02
mole = mass/molar mass
molar mass = mass/ mole
= 1.44/0.02
= 72 grams.
C5Hx = 72
12 × 5 + x = 72
x = 72 - 60
x = 12
An ideal gas is a theoretical gas composed of many randomly transferring factor particles that aren't difficult to interparticle interactions. the best gasoline idea is beneficial because it obeys the precise gas law, a simplified equation of country, and is amenable to evaluation under statistical mechanics.
An ideal gas is described as one for which both the extent of molecules and forces between the molecules are so small that they have got no effect on the behavior of the gas. The real gas that acts almost like a really perfect gasoline is helium. that is due to the fact helium, in contrast to maximum gases, exists as an unmarried atom, which makes the van der Waals dispersion forces as low as viable.
Learn more about ideal gas here:-brainly.com/question/20348074
#SPJ9
How many molecules are in 17 amu of ammonia, NH3?
A) 6.0 × 10²²
B) 1.2 × 10²²
C) 6.0 × 10²³
D) 1.2 × 10²³
Answers
Hopefully this helps
“16.2gAl x 1 mol Al/ 26.98gAl x 6.022 x 10^23 Al atoms/1 mol Al” and please explain
Answers
The number of atoms in 16.25 g of Al is calculated here. One mole or 26.98 g of Al contains 6.022 × 10²³ atoms. Thus, the atoms in 16.25 g or 0.60 mol of Al contains 3.62 × 10²³ atoms.
What is one mole ?Any substance containing 6.022 × 10²³ atoms is called one mole of that substance. This number is called Avogadro number. Therefore, one mole of every substances contains Avogadro number of atoms. The mass of one mole of an atom is called its atomic mass.
Al is 13th element in periodic table. Its atomic mass is 26.98 g/mol. Thus, the number of moles of 16.25 g of Al is calculated as follows:
no.of moles = given mass/atomic mass
= 16.25/26.98 =0.60 moles.
1 mole of Al contains 6.022 × 10²³ atoms. Thus, 0.60 moles contains 6.022 × 10²³ × 0.60 = 3.62 × 10²³ atoms.
Therefore, to calculate the number of atoms in a given number of moles of an element multiply the number of moles with Avogadro's number.
To find more on Avogadro number, refer here:
https://brainly.com/question/28834341
#SPJ1
at a high altitude water boils at 95'c instead of 100'c as at sea level because
having a definite orderly arrangement of particle
the atmospheric pressure is less
its vapor pressure drops
Answers
At a high altitude, water boils at a lower temperature than at sea level because the atmospheric pressure is lower. Option B
What happens at high altitude?The atmospheric pressure decreases with increasing altitude because there is less air pressing against the water's surface.
When a liquid's vapor pressure reaches the same level as air pressure, boiling takes place. At sea level, the atmospheric pressure is 1 atm, hence for water to boil at 100°C, the vapor pressure must also be 1 atm. In contrast, the vapor pressure of water exceeds atmospheric pressure at lower temperatures at high elevations when the atmospheric pressure is lower.
Learn more about pressure:https://brainly.com/question/31940213
#SPJ1
below are three aqueous solutions: 4cl solution 2: 0.15 m k2so4 solution 3: 0.25 m c6h12o6 answer the three following questions based upon the three solutions given. 1. which solution has the highest freezing point? 1 2. which solution has the highest vapor pressure? 2 3. which solution has the highest boiling point?
Answers
1. The solution with the highest freezing point is Solution 1 (4Cl).
This is because it has the lowest concentration of solute particles compared to the other solutions, resulting in less interference with the formation of the solid lattice structure of the solvent, thus a higher freezing point.
2. The solution with the highest vapor pressure is also Solution 1 (4Cl).
A lower concentration of solute particles means fewer solute-solvent interactions, allowing more solvent particles to escape into the vapor phase, leading to higher vapor pressure.
3. The solution with the highest boiling point is Solution 3 (0.25 M C6H12O6).
This is because it has the highest concentration of solute particles, resulting in a greater increase in boiling point due to increased solute-solvent interactions, which require more energy to break for the solvent to transition into the vapor phase.
to know more solutions refer here:
https://brainly.com/question/30665317#
#SPJ11
Benzene, C6H8, has an enthalpy of fusion = 10.19 kJ/mol. Calculate the amount of energy which is needed to change 88.0 g of solid benzene at 5.53°C into liquid benzene, also at 5.53°C?
Answers
Answer: About 10,200 Joules of heat is required to transform 80.0 g of solid benzene at 5.53°C into liquid benzene, also at 5.53°C.
1107 Joule is the amount of energy which is needed to change 88.0 g of solid benzene at 5.53°C into liquid benzene, also at 5.53°C.
What is energy?In order to perform work and to produce heat and light, energy must be delivered to a body or to an external physical system. Energy is a quantitative property. Energy is a preserved resource; according to the rule of conservation of energy, energy can only be transformed from one form to another and cannot be created or destroyed.
A moving object's kinetic energy, an object's potential energy, an object's elastic energy, chemical energy linked to chemical reactions, electromagnetic radiation's radiant energy, and the internal energy of a thermodynamic system are examples of common kinds of energy.
mole of benzene = 85.2/78.11 =1.09mol
1 mole of benzene requires 10.19 kJ/mol energy
1.09 mole of benzene requires 1.09× 10.19 kJ/mol = 11.107kJ/mol energy
= 1107 Joule energy
Therefore, 1107 Joule is the amount of energy which is needed to change 88.0 g of solid benzene at 5.53°C into liquid benzene, also at 5.53°C.
To learn more about energy, here:
https://brainly.com/question/29763772
#SPJ3
ANSWER ASAP please I need help thank you I’ll give you brainly
What is the standard cell notation of a galvanic cell made with silver (Ag) and
nickel (Ni)?
A. Ni2+(aq) | Ni(s) || Ag(s) | Ag+(aq)
B. Agt(aq) | Ag(s) || Ni(s) | Ni2+(aq)
C. Ag(s) | Ag+(aq) || Ni2+(aq) | Ni(s)
D. Ni(s) | Ni2+(aq) || Ag+(aq) | Ag(s)
Answers
Answer:
See the attached notation!
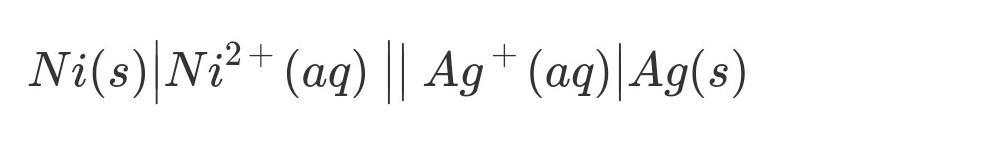
Need help in science earths moving surface

Answers
Answer:
All answers stated below:
Explanation:
1. Plate Tectonics
2. lithosphere
3. asthenosphere
4. divergent boundary
5. (not sure)
6. convergent
7. transform
8. convection
what is the wavelength (in nm) of the line in the spectrum of the hydrogen atom that arises from the transition of the electron from the orbital with n
Answers
Answer:
The wavelength of the line in the spectrum of the hydrogen atom is 102.57 nm.
The line in the spectrum of the hydrogen atom that results from the electron moving from the orbital with n = 5 to the orbital with n = 2 has a wavelength of 434 nanometers (nm).
The hydrogen atom is the most fundamental form of hydrogen. There is one proton, one electron, and no neutrons in the hydrogen atom. It's the lightest element on the periodic table, and it's also the most abundant. The symbol for hydrogen is H. It is the element that is present everywhere in the cosmos.
To know more about wavelength click here:
brainly.com/question/14286925
#SPJ11
Please help me in this chemistry question.

Answers
please what is the formula for finding mole and give a worked example
Answers
Answer:
see explanation
Explanation:
number of moles = mass ÷ relative formula mass
e.g.
Calculate the number of moles of carbon dioxide molecules in 22 g of CO2.
Ar (relative atomic mass) of C = 12, Ar of O = 16
Mr (relative formula mass) of carbon dioxide = 12 + 16 + 16 = 44
number of moles = 22 ÷ 44 = 0.5 mol
Metal or Non-metal?
Potassium: metal
Fluorine:
Bromine:
Hydrogen
Beryllium:
Nitrogen
Answers
Answer:
Fluorine: non-metal
Bromine: non-metal
Hydrogen: non-metal
Beryllium: metal
Nitrogen:non-metal
chemistry help please!!!

Answers
how many grams of methanol are produced when 7.0 grams of carbon monoxide reacts with 2.5 grams of hydrogen gas?
Answers
≈ 0.008 mol CH3OH
Hence the correct answer is 0.008 mol.
The statement of mass (grams) of methanol is "0.008 g."
What is mass?
A substance is something to do with mass that takes up space. The mass of a compound is the total amount of atoms present in grams that make up a molecule. The grams is the unit of mass.
The balanced equation comes out to be,
CO(g) + 2H2(g) → CH3OH(l)
In order to calculate the mass of methanol, initially the moles of the substance to be calculated from the given mass of CO and H2.
Gram to mole conversion,
To know more about methanol visit:
https://brainly.com/question/24077457
#SPJ4

Discuss why the following affect the rate of diffusion: molecular size, temperature, solution density, and the distance that must be traveled.
Answers
Molecular size: Molecules that are heavier than others move more slowly.
Temperature: The energy in the medium changes depending on whether the temperature is rising or falling, which has an impact on molecular movement.
Density: The harder it is for molecules to travel through a solution, the slower diffusion will be owing to density.
Distance: Diffusion is unable to transport nutrients and waste products efficiently enough to support life if the distance these substances must travel is too great.
What is diffusion?
Diffusion is the term for the net movement of anything, often from a location of higher concentration to one of lower concentration. A gradient in the chemical potential or Gibbs free energy is what drives diffusion.
To learn more about diffusion click the given link
https://brainly.com/question/94094
#SPJ4
Whats the answer marking brainliest :-)

Answers
Answer:
A
Explanation:
hope this helps you have a good day
supposed chemists attempt to produce an element with atomic number 119 based on it’s likely position on the periodic table what would you expect it’s electronegativity to be? explain how you can make this prediction
Answers
An element with atomic number 119 will be an alkali metal with a +1 oxidation state which makes it highly electronegative.
What is Electronegativity?This is described as the tendency of the atom of an element to attract electrons so as to form a bond. This is done so that the elements can achieve a stable octet configuration.
On the other hand, if an element with atomic number 119 was present based on it’s likely position on the periodic table then it will most likely be an alkali metal with a +1 oxidation state and will be highly electronegative as it requires the loss of only one electron in other to achieve a stable configuration thereby making it highly reactive.
Read more about Electronegativity here https://brainly.com/question/18258838
#SPJ1
do you think pokemon is bad
Answers
Use what you know about the orbits of Earth and the moon to sort the phrases into the correct Category.

Answers
\(\mathfrak{\huge{\orange{\underline{\underline{AnSwEr:-}}}}}\)
Actually Welcome to the Concept of the Astrophysics.
Let's categorize them eventually ==>
1.) Earth ==> 1.) travels around the Sun
2.) takes 365.25 days .
2.) Moon ===> 1.) travels around the Earth
2.) Takes 27.3 days.