Calculate the ph of a buffer solution prepared by mixing 30 ml of 5% acetic acid with 10 ml of 0. 5 m naoh.
Answers
The concentration of acetic acid is:[HA] = mass/volume= 31.5 g/0.04 L= 787.5 g/LTo calculate the pKa of acetic acid, we can use the formula:pKa = -log(Ka)The Ka (acid dissociation constant) of acetic acid is 1.8 x 10^-5.pKa = -log(1.8 x 10^-5) = 4.74Finally, we can substitute the values we have calculated into the equation for pH:pH = 4.74 + log(0.125/787.5)pH = 4.74 - 1.49pH = 3.25Therefore, the pH of the buffer solution is 3.25.
A buffer solution is a solution that maintains a constant pH level despite the addition of an acid or base. Buffer solutions are generally composed of a weak acid and its corresponding conjugate base, or a weak base and its corresponding conjugate acid.In the given problem, a buffer solution has been prepared by mixing 30 ml of 5% acetic acid with 10 ml of 0.5 M NaOH. The balanced chemical equation for the neutralization of acetic acid with NaOH is:CH3COOH + NaOH → CH3COONa + H2OIn order to determine the pH of this buffer solution, we need to first find the concentration of acetic acid and its conjugate base. Let's start with acetic acid.The density of 5% acetic acid is 1.05 g/mL. Therefore, the mass of 30 mL of 5% acetic acid can be calculated as follows:mass = density x volume= 1.05 g/mL x 30 mL= 31.5 gNext, we can calculate the number of moles of acetic acid present in the solution using its molar mass.Molar mass of acetic acid (CH3COOH) = 60.05 g/molNumber of moles of acetic acid = mass/molar mass= 31.5 g/60.05 g/mol= 0.525 molNow, we can use the formula for calculating the pH of a buffer solution:pH = pKa + log([A-]/[HA])Where:pKa = the negative logarithm of the acid dissociation constant ([H+][A-]/[HA])[A-] = concentration of the conjugate base (acetate ion, CH3COO-) in moles/L[HA] = concentration of the weak acid (acetic acid, CH3COOH) in moles/LTo calculate the concentration of acetate ion, we need to know the number of moles of NaOH that reacted with acetic acid. Since the concentration of NaOH is 0.5 M, we can calculate the number of moles of NaOH as follows:Number of moles of NaOH = concentration x volume= 0.5 mol/L x 0.01 L= 0.005 molSince NaOH reacts with acetic acid in a 1:1 ratio, the number of moles of acetate ion produced is also 0.005 mol. The total volume of the buffer solution is 30 mL + 10 mL = 40 mL, or 0.04 L. Therefore, the concentration of acetate ion is:[A-] = number of moles/volume= 0.005 mol/0.04 L= 0.125 mol/LTo calculate the concentration of acetic acid, we need to know the initial concentration of acetic acid and the volume of the solution. The initial concentration of acetic acid is 5% or 0.05 g/mL. The mass of acetic acid in 30 mL of 5% solution is 31.5 g (calculated earlier). The volume of the buffer solution is 0.04 L. Therefore, the concentration of acetic acid is:[HA] = mass/volume= 31.5 g/0.04 L= 787.5 g/LTo calculate the pKa of acetic acid, we can use the formula:pKa = -log(Ka)The Ka (acid dissociation constant) of acetic acid is 1.8 x 10^-5.pKa = -log(1.8 x 10^-5) = 4.74Finally, we can substitute the values we have calculated into the equation for pH:pH = 4.74 + log(0.125/787.5)pH = 4.74 - 1.49pH = 3.25Therefore, the pH of the buffer solution is 3.25.
To Know more about pH visit:
brainly.com/question/2288405
#SPJ11
Related Questions
what is a common rock that is readily dissolved by water and weak acids? all of these choices are correct.quartz-rich sandstonequartzitelimestone
Answers
Limestone is a common rock that is readily dissolved by water and weak acids due to its calcium carbonate composition.
Limestone is a typical stone that is promptly broken up by water and feeble acids. It is a sedimentary stone essentially made out of calcium carbonate, which responds with feeble acids, remembering carbonic corrosive present for water and soil, prompting synthetic enduring and disintegration.
The disintegration of limestone structures different elements like caverns, sinkholes, and underground seepage frameworks. This rock type is far and wide and is shaped in shallow, warm marine conditions by the collection of natural flotsam and jetsam and calcium carbonate minerals.
The solidness and porosity of limestone make it a famous material for development and structural purposes, however its dissolvability can prompt issues like sinkholes, subsidence, and groundwater tainting. Generally, limestone is a significant stone sort in geographical and ecological settings because of its extraordinary properties and powerlessness to substance enduring.
To learn more about common rock, refer:
https://brainly.com/question/28199073
#SPJ4
The complete question is:
What is a common rock that can be dissolved by water and weak acids?
A. quartzite
B. quartz-rich sandstone
C. limestone
D. all of these
Protons and neutrons are found in ____________ part of an atom.
Answers
Protons and neutrons are found in nucleus part of an atom.
The nucleus or the center of an atom is made up of the protons and the neutrons. The number of the protons in the nucleus and it is known as the atomic number. This primarily will determines where that atom will fits on the Periodic Table.
The nucleus is the small and the dense region that is consisting of the protons and the neutrons at the center of the atom. Protons are the type of the subatomic particle with the positive charge. Protons is bound together in the atom's nucleus as the result of the strong nuclear force.
To learn more about protons here
https://brainly.com/question/29248303
#SPJ4
consider the compounds cl2, hcl, f2, naf, and hf. which compound has a boiling point closest to that of argon? explain.
Answers
The compound that has a boiling point closest to that of Argon is HF. This is because HF has the strongest intermolecular forces (hydrogen bonding) among the given compounds.
The boiling point of a compound depends on the strength of the intermolecular forces that exist between the molecules. The stronger the intermolecular forces, the higher the boiling point.
The weaker the intermolecular forces, the lower the boiling point. The boiling point of Argon is -186°C. Out of the given compounds, the boiling point of HF is the closest to the boiling point of Argon.
The boiling point of HF is -83.8°C. This is because HF has hydrogen bonding which is the strongest intermolecular force among the given compounds. The other compounds such as Cl2, F2, HCl, and NaF, have weaker intermolecular forces than HF. Therefore, they have a lower boiling point than HF.
Learn more about HF here:
https://brainly.com/question/14581360#
#SPJ11
you consider starting material a. you know that a can undergo two irreversible reactions as shown in the below reaction coordinate diagram with one reaction pathway labeled in red and one reaction pathway labeled in blue. the red path leads to product b, while the blue path leads to product c. assuming both reaction pathways occur simultaneously in competition with each other, what is the major product, and why?
Answers
Product B because it has a lower energy level than Product C's transition state, which leads to Product C.
What are reaction pathways?The series of reactions required to create a desired product are described by a reaction pathway. The distribution strategy for a product is determined by things like percentage yield. Atomic economics. reaction time. is a connected graph with chemical species as its nodes. If a reaction transfers material from one species to the other, an edge unites the two. An vector from reactant toward the product is depicted as the edge.
What role do reactions pathway ?Energy, or ATP, is created by chemical reactions within our cells. All living things require energy to survive, and Adp would be a reactant that fuels a number of other chemical reactions inside cells. Cells generate energy through a process called cellular respiration.
To know more about Reaction pathways visit:
https://brainly.com/question/16047003
#SPJ4
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
In the Haber Process, ammonia is synthesized from nitrogen andhydrogen:
N2 (g) + 3H2 -----> 2NH3(g)
ΔG at 298K for this reaction is -33.3 kj/mol. the valuef ΔG at 298 K for a reaction mixture that consists of 1.9 atmN2, 1.6 atm H2 and 0.65 atm NH3 is________.
a.) -3.86 x 103
b.) -1.8
c.) -7.25 x 103
d.) -40.5
e.) -104.5
Answers
The value of ΔG at 298 K for a reaction mixture containing 1.9 atm N2, 1.6 atm H2, and 0.65 atm, the answer is (a) -3.86 × 10^3.
NH3 can be calculated using the equation:
ΔG = ΔG° + RT ln(Q)
where ΔG is the standard Gibbs free energy change, ΔG° is the standard Gibbs free energy change at standard conditions, R is the gas constant, T is the temperature in Kelvin, and Q is the reaction quotient.
In this case, we are given ΔG° as -33.3 kJ/mol. To calculate Q, we need to use the partial pressures of the gases in the reaction mixture. The reaction stoichiometry tells us that the ratio of the partial pressures of N2, H2, and NH3 is 1:3:2. Therefore, we can write:
Q = (P(NH3))^2 / (P(N2) * P(H2)^3)
Plugging in the given values of P(N2) = 1.9 atm, P(H2) = 1.6 atm, and P(NH3) = 0.65 atm, we can calculate Q. Then, using the value of R = 8.314 J/(mol·K) and the temperature T = 298 K, we can substitute these values into the equation and solve for ΔG.
The calculated value of ΔG at 298 K for the given reaction mixture is approximately -3.86 × 10^3 J/mol. This value is equivalent to -3.86 kJ/mol. Therefore, the answer is (a) -3.86 × 10^3.
To learn more about Haber Process here brainly.com/question/30928282
#SPJ11
determine the solubility of kcl at 60 °c in 100g of h2o?
Answers
The solubility of KCl at 60 °C in 100 g of water (H2O) can be determined using experimental data or by using a solubility table. The solubility of a substance refers to the maximum amount of that substance that can dissolve in a given amount of solvent at a particular temperature and pressure.
One possible way to determine the solubility of KCl at 60 °C in 100 g of water is to consult a solubility table, which lists the solubility of various substances in water at different temperatures. According to one such table, the solubility of KCl in water at 60 °C is approximately 47 g per 100 g of water.
This means that 100 g of water at 60 °C can dissolve up to 47 g of KCl before becoming saturated, i.e., no more KCl will dissolve in the water at this temperature.
It is important to note that the solubility of KCl (or any substance) in water can be affected by various factors, such as temperature, pressure, and the presence of other solutes. Therefore, the solubility value obtained from a solubility table is only an approximation and may not be accurate for all conditions.
For more details about solubility click here:
https://brainly.com/question/29661360#
#SPJ11
65 million years ago there was a mass extinction that is best known for killing the dinosaurs. There was also a large
deposit of dust that contained substances only found in asteroids, What most likely killed off the dinosaurs?
Answers
Answer:
65 million years ago there was a mass extinction that is best known for killing the dinosaurs. There was also a large
deposit of dust that contained substances only found in asteroids, What most likely killed off the dinosaurs?
Explanation:
Answer:
b
Explanation:
What determines the order of amino acids in a protein?
A. The order of nucleotide bases within a gene
B. Enzymes present at the ribosomes
C. Amino acids available in cell cytoplasm
Answers
A. The order of nucleotide bases within a gene determines the arrangement of amino acids in a protein. The genetic code, which is made up of the bases in DNA, dictates the order in which the amino acids will be combined to form proteins.
The DNA nucleotide base sequence is translated into a complementary sequence of bases in mRNA during the protein production process. Then, using the mRNA as a template, a protein chain is put together, with each triplet of bases (known as a codon) designating a certain amino acid. The ribosomes, which are in charge of protein synthesis, assemble the amino acids in the order dictated by the mRNA rather than choosing the amino acid sequence of a protein. Although necessary for protein synthesis, the availability of amino acids in the cell cytoplasm does not affect the precise amino acid sequence of a protein.
Learn more about Amino acids here:
https://brainly.com/question/14583479
#SPJ4
A shopper in a supermarket pushes a cart with a force of 35 N directed at
an angle of 25° downward from the horizontal. Find the work done by the
shopper on the cart as the shopper moves along a 50. 0 m length of aisle.
Answers
The shopper does approximately 1580.5 Joules of work on the cart as they move along the 50.0 m length of the aisle.
To find the work done by the shopper on the cart, we can use the formula: work = force * distance * cos(angle).
Given:
Force (F) = 35 N
Angle (θ) = 25° downward from the horizontal
Distance (d) = 50.0 m
First, we need to determine the horizontal component of the force, which is F_horizontal = F * cos(θ).
F_horizontal = 35 N * cos(25°) ≈ 31.61 N
The work done by the shopper on the cart is then:
Work = F_horizontal * distance
Work = 31.61 N * 50.0 m = 1580.5 Joules
Therefore, the shopper does approximately 1580.5 Joules of work on the cart as they move along the 50.0 m length of the aisle.
For more question on work
https://brainly.com/question/26487364
#SPJ8
Dental implant data: The hardness of metal implant in dental cavities depends on multiplefactors, such as the method of implant, the temperature at which the metal is treated, thealloy used as well as on the dentists who may favour one method above another and maywork better in his/her favourite method. The response is the variable of interest.
Answers
The hardness of metal dental implants depends on implant method, treatment temperature, alloy used, and dentist expertise.
The hardness of a metal implant in dental cavities is influenced by various factors. Firstly, the method of implantation plays a crucial role. Different techniques, such as immediate placement or two-stage placement, can affect the final hardness of the implant.
Secondly, the temperature at which the metal is treated during fabrication and processing can impact its hardness. Higher temperatures can lead to improved hardness properties.
Moreover, the choice of alloy used for the implant material is significant. Different alloys, such as titanium or zirconia, possess varying hardness characteristics, affecting the overall hardness of the implant.
Dentists may have their preferences for specific alloys based on their experiences and patient needs.
Lastly, the skill and technique of the dentist performing the implantation procedure can influence the hardness outcome. Dentists who are proficient and experienced in a particular method may achieve better results in terms of hardness.
In summary, the hardness of a metal implant in dental cavities depends on factors like the implantation method, temperature during treatment, choice of alloy, and the expertise of the dentist.
Understanding and optimizing these factors can contribute to achieving desirable hardness properties for dental implants.
Learn more about Implants
brainly.com/question/31834956
#SPJ11
Someone makes a loud noise, and it scares you. Use complete sentences to put the following vocabulary terms in order to describe how the body processes the sound and reacts: central nervous system, sensory organs, and sensory receptors.
Answers
The vibrating particles are collected by the sensory receptors which are found in the sense organ(ear) to the central nervous system(brain) for processing.
What is Sense organ?There are five types which include the following:
EyesEarsNoseTongueSkin.They have receptors which transport stimuli to the central nervous system for processing.
Read more about Sense organs here https://brainly.com/question/24457504
What mass of water freezes if 5.43 kJ oh heat are released in the process
Answers
Answer:
16.3 g
Explanation:
got it right on ck
Ammonia (NH3) decomposes to hydrogen ( H2) and nitrogen (N2) and 22.0 kcal/mol of energy was
absorbed as shown in the balanced equation below:
2 NH3(g) 3H2(g) + N2 (g) ΔH = + 22.0 kcal/mole
List the conversion factors for this reaction.
a. How many kilocalories of energy will be absorbed if 15.5 moles of ammonia (NH3) was decomposed?
b. How much energy is absorbed when 250.5 grams of hydrogen H2) are produced?
c. How much energy will be absorbed in order to produce 1530.0 grams of nit
d. How many grams of ammonia (NH3) should be decomposed in order to release 5500 kilocalories of energy?
Answers
To release 5500 kilocalories of energy, approximately 5500 grams of NH₃ should be decomposed.
The conversion factors for the given reaction are:
1 mole of NH₃ produces 3 moles of H₂ and 1 mole of N₂.
ΔH = + 22.0 kcal/mol (energy absorbed per mole of NH₃ decomposed).
a. To calculate the kilocalories of energy absorbed when 15.5 moles of NH₃ is decomposed:
Energy absorbed = ΔH * moles of NH₃
Energy absorbed = 22.0 kcal/mol * 15.5 mol = 341.0 kcal
b. To determine the energy absorbed when 250.5 grams of H₂ are produced, we need to convert the mass of H₂ to moles using its molar mass:
Molar mass of H₂ = 2.02 g/mol
Moles of H₂ = Mass / Molar mass = 250.5 g / 2.02 g/mol = 124.0 mol (approximately)
Energy absorbed = ΔH * moles of NH₃
Energy absorbed = 22.0 kcal/mol * 124.0 mol = 2,728.0 kcal
c. To find the energy absorbed in order to produce 1530.0 grams of N₂, we need to convert the mass of N₂ to moles using its molar mass:
Molar mass of N₂ = 28.02 g/mol
Moles of N₂ = Mass / Molar mass = 1530.0 g / 28.02 g/mol = 54.68 mol (approximately)
Energy absorbed = ΔH * moles of NH₃
Energy absorbed = 22.0 kcal/mol * 54.68 mol = 1,202.96 kcal (approximately)
d. The grams of NH₃ needed to release 5500 kilocalories of energy, we can rearrange the equation:
Grams of NH₃ = Energy released / (ΔH * moles of NH₃)
Grams of NH₃ = 5500 kcal / (22.0 kcal/mol * 1 mol)
Grams of NH₃ = 5500 g (since 1 kcal = 1 g)
To learn more about kilocalories refer here:
https://brainly.com/question/30667858#
#SPJ11
Describe how magnesium nitrate crystals can be obtained from a solution
Answers
Answer:
magnesium carbonate reacts with aqueous acids to release carbon dioxide and water
MgCO3 + 2 HCl → MgCl2 + CO2 + H2O.
How to -
Step 1: Reaction
- Leave the dilute hydrochloric acid in a beaker.
- Add Magnesium carbonate slowly until it is in excess or until no more gas seem to be getting liberated.
Step 2: Filtration
- Filter with filter paper and funnel.
- Filter off the excess magnesium carbonate as magnesium chloride will be in aqueous form (liquid) and will come out with the filtrate. The residue is the excess magnesium carbonate.
Step 3: Crystallization to obtain solid crystals from the filtrate.
- Pour filtrate solution into evaporating dish/basin
- Provide heat using Bunsen burner
- Pour solution into an evaporating basin and heat over a water bath
- Stop heating when crystals start to form
allow water to evaporate until pure crystals remain.
- Dry crystals using absorbent paper or warm oven.
Precautions
- Use personal protective equipment such as gloves, a lab coat and wear eye protection, especially when heating.
- Avoid inhaling unnecessary gases during the whole process.
Mark me as brainliest
Describe the natural processes which remove carbon dioxide from the atmosphere
Answers
Answer:
Explanation:
The process is photosynthesis as plants need carbon dioxide and humans need oxygen so plants release oxygen which humans breath/take in and plant/trees take in carbon dioxide which humans release.
The natural process which removes carbon dioxide from the atmosphere is photosynthesis.
What is photosynthesis?
It is defined as a process by which plants and other photosynthetic organisms convert the light energy in to chemical energy through the process of cellular respiration.
Some of the energy which is converted is stored in molecules of carbohydrates like sugar and starches which are made up of from carbon dioxide and water . Photosynthetic organisms which can perform photosynthesis are algae and cyanobacteria. Photosynthesis is largely responsible for producing and maintaining the content of oxygen in earth's atmosphere.
The process begins with proteins absorbing light energy which are called reaction centers and contain a green pigment which is called chlorophyll . In plants ,these pigments are present inside organelles called chloroplasts while in bacteria they are present in plasma membrane.
Learn more about photosynthesis,here:
https://brainly.com/question/29764662
#SPJ2
What is the concentration if 0.25 mil is dissolved in 500cm cubed of solution
Answers
Answer:
0.5 mol/dm3
Explanation:
e.g. g/dm3, g/cm3 and mol/dm3
= g dm-3, g cm-3 and mol dm-3
hhhhhhhhhhhheeeeeeeelpppppp

Answers
Answer:
Solar photons
Hope this helps :)
Diffusion in Solids It is desired to calculate the rate of diffusion of CO₂ gas in air through a loosely packed bed of sand at 276K and a total pressure of 1 atm. The bed depth is 1.25 m and the void fraction e is 0.3. The partial pressure of CO₂ at the top of the bed is 2.026 x 10' Pa and 0 Pa at the bottom. Assume equimolar counterdiffusion of CO₂ and air. Use a t of 1.87. DAB-0.142×10 m²/s.
Answers
the rate of diffusion of CO₂ gas in air through the bed of sand is approximately 2.304 × 10^-6 mol/(m²·s).
To calculate the rate of diffusion of CO₂ gas in air through a bed of sand, we can use Fick's law of diffusion:
J = -DAB (dC/dx)
where J is the molar flux of CO₂, DAB is the diffusion coefficient of CO₂ in air, and (dC/dx) is the concentration gradient of CO₂ in the direction of diffusion.
To calculate the concentration gradient, we can use the following equation:
(dC/dx) = (ΔC/Δx)
where ΔC is the difference in partial pressure of CO₂ between the top and bottom of the bed, and Δx is the bed depth.
We are given that the bed depth is 1.25 m and the void fraction is 0.3, which means that the volume of the bed is:
V = (1 - e) A L
where A is the cross-sectional area of the bed and L is the bed depth. Assuming a circular cross-section, we can calculate the area as:
A = π (d/2)²
where d is the diameter of the bed. We are not given the diameter, so we cannot calculate the area.
However, we are given the partial pressure of CO₂ at the top and bottom of the bed, as well as the diffusion coefficient and temperature. We can use these values to calculate the molar flux of CO₂ using Fick's law of diffusion.
First, we need to convert the diffusion coefficient to the appropriate units:
DAB = 0.142 × 10^-9 m²/s
Next, we can calculate the concentration gradient:
ΔC = 2.026 × 10^4 Pa - 0 Pa = 2.026 × 10^4 Pa
Δx = 1.25 m
(dC/dx) = (ΔC/Δx) = (2.026 × 10^4 Pa/1.25 m) = 1.6208 × 10^4 Pa/m
Finally, we can calculate the molar flux of CO₂:
J = -DAB (dC/dx) = -(0.142 × 10^-9 m²/s) (1.6208 × 10^4 Pa/m) = -2.304 × 10^-6 mol/(m²·s)
The negative sign indicates that the molar flux of CO₂ is in the opposite direction of the concentration gradient, which is expected for equimolar counterdiffusion.
Therefore, the rate of diffusion of CO₂ gas in air through the bed of sand is approximately 2.304 × 10^-6 mol/(m²·s).
please help me with honors chem I will give brainliest answer
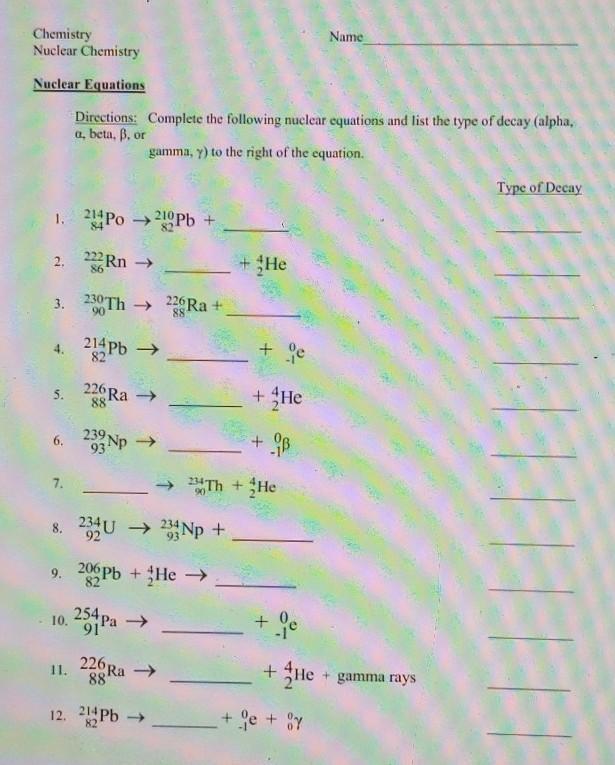
Answers
Answer:
see the pic for the answer


PLEASE IM VERY CONFUSED
Directions: Write the formulas of the reactants and products, including the symbols for the state,
(s), (l), (g), (aq) - then balance the equations.
8. When a solution of hydrogen chloride is added to solid sodium bicarbonate (NaHCO3), the products are carbon dioxide, water and aqueous sodium chloride.
9. Ethyl alcohol (a liquid), C2H6O, burns in air to produce carbon dioxide and gaseous water.
10. Solid titanium (IV) chloride reacts with water, forming solid titanium (IV) oxide and aqueous hydrogen chloride.
11. During photosynthesis in plants, carbon dioxide and water are converted into glucose, C6H12O6, and oxygen gas.
12. Solutions of calcium hydroxide, Ca(OH)2 and nitric acid, HNO3, react to produce water and aqueous calcium nitrate, Ca(NO3)2.
Answers
Answer:
8. the formula of the products are CO2(g) , H2O (l) and NaCl (aq)
Explanation:
s- solid
g- gas
l- liquid
aq- aqueous, means acid
These are the products of the equation, you can balance this by searching up a video on how to do so, or use a balance equations calculator online. Balancing is very easy tho.
explain why the water cycle is repeated
Answers
Answer:
Because as water is used it returns to the ground where it's evaporated by the sun and comes back to us as rain
Answer:
After the rain falls to earth, it may stay here for a long time. Some water stays underground among the rocks for thousands of years. Eventually, however, the water will end up someplace where it can be evaporated, often in the ocean, and then the water cycle repeats itself.
:))
Which of the following object has the LESS momentum?
a train with a velocity of 0 m/s
or
a grasshopper with a velocity of 1 m/s
Answers
2Cr^3 + 3Zn(s) -> 2Cr(s) + 3Zn^2 + (aq) which reactant is reduced and which is oxidicided
Answers
Answer:
Cr is oxidised
and
Zn is reduced
[ Reduced reactant ] :
Cr^( + 3 ) + 3 e^( - 1 ) ===》 Cr^( 0 )
_____________________________
[ Oxidicided reactant ] :
Zn^(0) ===》Zn^( + 2 ) + 2 e^( - 1 )
10. How many moles of CuCl, are in 150 L of a 2.33 M Cuci, solution?
Answers
Answer:
349.5 moles
Explanation:
(I am assuming that there is a typo at the end, where Cuci solution = CuCl solution)
The formula for molarity is:
Molarity = moles in solution / volume of solution
Here, we are given both the Molarity and the volume. So we can plug it into the equation above and get:
2.33 = moles / 150
Now we solve for the moles by doing:
moles = 2.33 * 150
This gives us the answer of 349.5 moles.
Why is important for your scientific observations to be as detailed as possible?
Answers
Answer:
They must be detailed enough that another scientist can repeat exactly what you did and know if they are getting the same data. ... To make sure that data can be reproduced by other people and other laboratories. Also so that you know exactly what is there and to know you arn't missing anything. ~ Hope this helps :>
Explanation:
nếu có 40g dung dịch NaOH 20% phairn dùng hết bao nhiêu gam dung dịch HCl 25% để trung hoà
Answers
Answer:
nếu có 40g dung dịch NaOH 20% phairn dùng hết bao nhiêu gam dung dịch HCl 25% để trung hoà
Is petrol a solvent
Answers
Answer: yes
Explanation: Petroleum solvents are hydrocarbon mixtures which can be grouped into three broad categories on the basis of their boiling ranges and solvent strengths, as follows: special boiling range solvents, boiling range, 30-160 oC; white spirits, 130-220 oC; and high-boiling aromatic solvents, 160-300 oC.
The energy required to remove an electron from the outermost shell is called
Answers
Answer:
Ionization energy
Explanation:
Ionization energy can be defined as the energy needed to remove an electron from the outermost she'll.
Give the valencies of metals X,Y and Z and are 1,2,3 respectively what are the formulae of of their hydrogen carbonate
Answers
the formulae of the hydrogen carbonates of metals X, Y, and Z are X(HCO3), Y(HCO3)2, and Z(HCO3)3, respectively. The valencies of metals X, Y, and Z are 1, 2, and 3 respectively.
The formula for hydrogen carbonate is HCO3-, which consists of one hydrogen ion, one carbonate ion, and two oxygen atoms.To determine the formula for the hydrogen carbonates of metals X, Y, and Z, we need to consider the charges of the metal cations and the carbonate anion. For X with a valency of 1, the formula for its hydrogen carbonate is X(HCO3), where X is the metal cation with a charge of +1. For Y with a valency of 2, the formula for its hydrogen carbonate is Y(HCO3)2, where Y is the metal cation with a charge of +2. For Z with a valency of 3, the formula for its hydrogen carbonate is Z(HCO3)3, where Z is the metal cation with a charge of +3.
learn more about hydrogen carbonates of metals here:
https://brainly.com/question/28246767
#SPJ11