Answers
Answer:
pH = 0.18
Explanation:
The sulfuric acid (H₂SO₄) has the following reactions in aqueous medium:
H₂SO₄ → HSO₄⁻ + H⁺
HSO₄⁻ ⇄ SO₄²⁻ + H⁺ Ka = 1.1x10⁻²
Where Ka is defined as Ka = [SO₄²⁻] [H⁺] / [HSO₄⁻] = 1.1x10⁻²
Based in the first reaction, [H⁺] = 0.65M and [HSO₄⁻] = 0.65M
In the second reaction, the two species are in equilibrium, thus, concentrations will be:
[H⁺] = 0.65M + X
[HSO₄⁻] = 0.65M - X
[SO₄²⁻] = X
Replacing in Ka formula:
1.1x10⁻² = [X] [0.65 + X] / [0.65M - X]
7.15x10⁻³ - 1.1x10⁻²X = 0.65X + X²
0 = X² + 0.661X - 7.15x10⁻³
Solving for X:
X = -0.67M → False solution. There is no negative concentrations.
X = 0.01065M → Right answer.
Thus [H⁺] = 0.65M + 0.01065M = 0.66065M
As pH = -log [H⁺];
pH = -log 0.66065M = 0.18
Related Questions
During a volcanic eruption, lava flowed at a rate of 37 m/min. At this rate how far in kilometers
can lava travel in 45 minutes?
Answers
does anyone know why electron affinity increases as going upward in the periodic table
Answers
Answer:
Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus, thus a stronger attraction between the nucleus and its electrons.
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
If Tracy were to put the pizza on the countertop to cool down, which temperature do you predict the pizza would get to?
a. -1 degrees Celsius
b. 10 degree Celsius
c. 27 degree Celsius
d. 93 degree Celsius
e. 260 degree Celsius
Answers
Answer:
d. 93 degree Celsius
Explanation:
Plzzzzzzzzzzzzzzzzzzzzzzz mark AS BRAINLIST
Which of the following are examples of physical properties of ethanol? Select all that apply.
The boiling point is 78.37°C
It is a clear, colorless liquid
It is flammable
It is a liquid at room temperature
Answers
Given the balanced equation representing a reaction at 101.3 kPa and 298 K:
N2(g) + 3H2(g) --> 2NH3(g) + 91.8 kJ
Which statement is true about this reaction?
Answers
The statement that is true is that It is endothermic and DH equals +91.8 kJ.
What is the truth?We can see that in this case that the heat of the reaction which we cam also call the enthalpy of the reaction is positive. Let us know that when we have a positive enthalpy what we have is an endothermic reaction.
Endothermic reactions are typically observed as a decrease in temperature, as energy is absorbed from the surroundings to fuel the reaction. Examples of endothermic reactions include the melting of ice.
Learn more about endothermic reaction:https://brainly.com/question/28984750
#SPJ1
Missing parts;
Given the balanced equation representing a reaction at 101.3 kPa and 298 K:
N2(g) + 3H2(g) => 2NH3(g) + 91.8 kJ
Which statement is true about this reaction?
(1) It is exothermic and DH equals -91.8 kJ.
(2) It is exothermic and DH equals +91.8 kJ.
(3) It is endothermic and DH equals -91.8 kJ.
(4) It is endothermic and DH equals +91.8 kJ.
A 300.0 mL quantity of hydrogen is collected over water at 19.5 C and a total atmospheric pressure of 750. mm Hg. The partial pressure of water at this temperature is 17.0 mm Hg
Answers
The partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg (calculated by subtracting the partial pressure of water, 17.0 mm Hg, from the total atmospheric pressure, 750.0 mm Hg).
When a gas is collected over water, the presence of water vapor affects the total pressure observed. In this case, the total atmospheric pressure is given as 750.0 mm Hg, and the partial pressure of water vapor at 19.5°C is 17.0 mm Hg.
To determine the partial pressure of hydrogen, we need to subtract the partial pressure of water vapor from the total atmospheric pressure. Partial pressure refers to the pressure exerted by an individual gas component in a mixture. In this scenario, the collected gas is primarily hydrogen, with water vapor being the other component.
By subtracting the partial pressure of water vapor (17.0 mm Hg) from the total atmospheric pressure (750.0 mm Hg), we can find the partial pressure of hydrogen:
Partial pressure of hydrogen = Total atmospheric pressure - Partial pressure of water vapor
Partial pressure of hydrogen = 750.0 mm Hg - 17.0 mm Hg
Partial pressure of hydrogen = 733.0 mm Hg
Therefore, the partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg.
Know more about hydrogen here:
https://brainly.com/question/24433860
#SPJ8
Calculate the standard cell potential, ∘cell, for the equation Co(s)+F2(g)⟶Co2+(aq)+2F−(aq) Use the table of standard reduction potentials. ∘cell=
Answers
Answer:
3.15 V
Explanation:
Step 1: Write the balanced cell reaction
Co(s) + F₂(g) ⟶ Co²⁺(aq) + 2 F⁻(aq)
Step 2: Identify both half-reactions:
Cathode (reduction): F₂(g) + 2 e⁻ ⟶ 2 F⁻(aq) E°red = 2.87 V
Anode (oxidation): Co(s) ⟶ Co²⁺(aq) + 2 e⁻ E°red = -0.28 V
Step 2: Calculate the standard cell potential
We will use the following expression.
E°cell = E°red,cathode - E°red,anode
E°cell = 2.87 V - (-0.28 V) = 3.15 V
Given E°cell > 0, the reaction is spontaneous.
Taking into account the definition of standard cell potential, E°cell has a value of 3.15 V.
The balanced cell reaction is:
Co(s) + F₂(g) ⟶ Co²⁺(aq) + 2 F⁻(aq)
First of all, it must be taken into account that oxidation is a reaction where an atom, ion or molecule loses electrons while reduction corresponds to the gain of electrons from an atom, ion or molecule.
Both oxidation and reduction depend on the change in the oxidation state of the atom, that is, on the difference in the charge of the atom in a reaction.
The oxidation and reduction reactions always occur simultaneously for what are generally known as oxidation-reduction reactions or redox reactions.
So, the anode corresponds to the negative electrode which normally oxidizes in the electrolytic chemical reaction while the cathode corresponds to the positive electrode which normally reduces its oxidation state when it receives electrons.
Si, in this case, the both half-reactions will be:
Cathode (reduction): F₂(g) + 2 e⁻ ⟶ 2 F⁻(aq) E°red = 2.87 V Anode (oxidation): Co(s) ⟶ Co²⁺(aq) + 2 e⁻ E°red = -0.28 V
The potential of an Ecel cell is a measure of the difference in electronic energy between the two electrodes. The electronic energy of each electrode is related to the force with which the reaction occurs at the electrode-solution interface. The unit in which it is measured is the volt.
In electrochemistry, because a cell reaction is made up of two half-cell reactions, each of which has a characteristic electrode potential, the potentials measure the driving force of the two half-reactions.
The cell potential is obtained by subtracting the potentials of both half cells as shown below:
E°cell= Ecatode - Eanode
So, in this case, the Ecell is calculated as:
E°cell = 2.87 V - (-0.28 V) = 3.15 V
In summary, E°cell has a value of 3.15 V.
Learn more:
https://brainly.com/question/14011488?referrer=searchResultshttps://brainly.com/question/13892163?referrer=searchResultshttps://brainly.com/question/17186824?referrer=searchResultshttps://brainly.com/question/13000465?referrer=searchResultshttps://brainly.com/question/12990827?referrer=searchResultsCan anyone help please.......
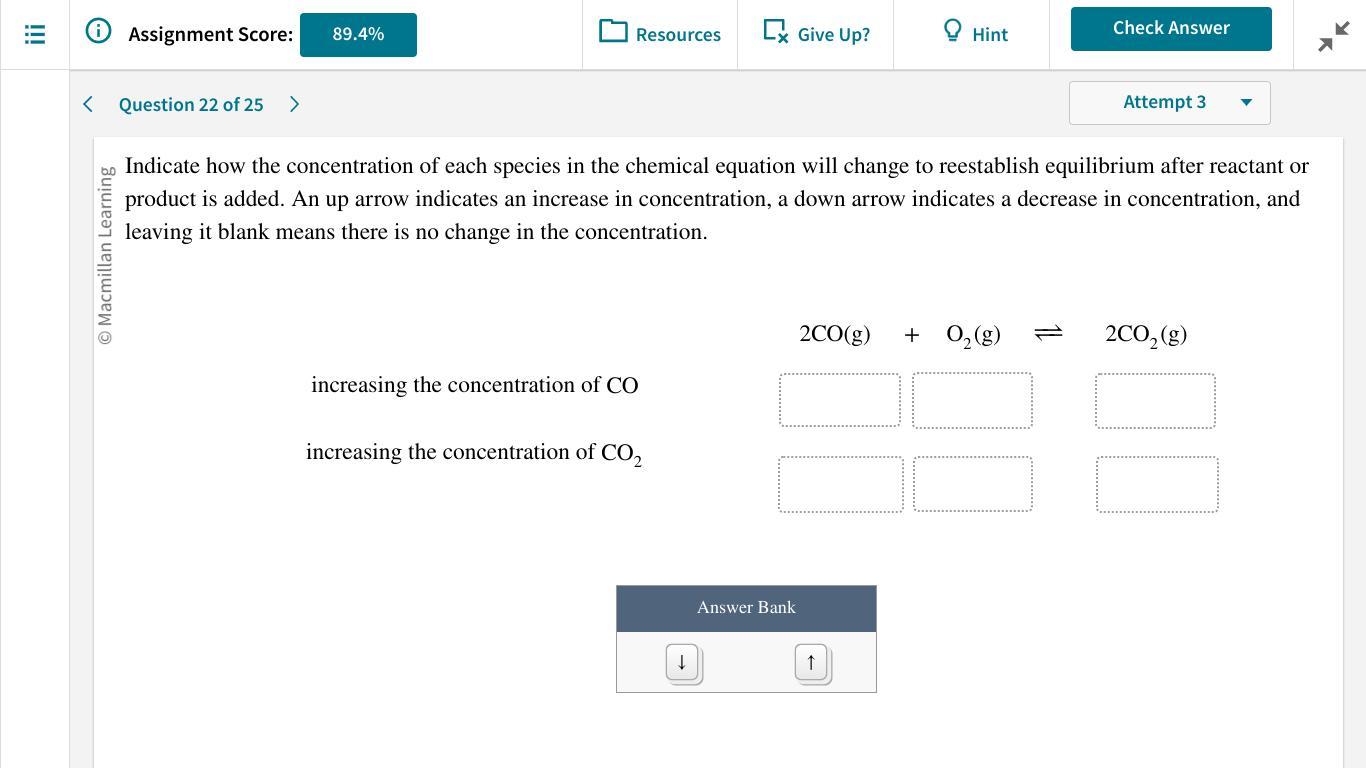
Answers
Increasing the concentration of CO decreases the equilibrium concentration of oxygen and increases the concentration of CO₂, increasing the concentration of CO₂ increases the concentration of CO and O₂.
Chemical equilibrium refers to the state of a system in which the concentration of the reactant and the concentration of the products do not change with time, and the system does not display any further change in properties.
It is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. While a reaction is in equilibrium the concentration of the reactants and products are constant.
Learn more about Equilibrium, here:
https://brainly.com/question/30985040
#SPJ1
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems.
A)
–1.68 V
B)
1.68 V
C)
0.78 V
D)
–0.78 V

Answers
Answer:
C: 0.78 V
Explanation:
The Cr2O7 cell has a higher reduction potential, so it will be reduced (as it is a better oxidizing agent). This means that Fe will be oxidized. Its oxidation potential is simply the negative of its reduction potential, 0.45: \(E_{ox}=-0.45\). We know that cell potential is the sum of the reduction and oxidation potentials of each reaction (it is not an extensive property like enthalpy or entropy, so we don't need to worry about multiplying anything). Therefore, \(E_{cell}=(-0.45+1.23)V=0.78V\).
What is the ΔG (kJ/mol) for a reaction at 25 Celsius that is:
Mg3(PO4)2 (s) ⇄ 3 Mg2+ (aq) + 2 PO43− (aq) ΔG0 = 137.0 kJ/mol
If there is initially 0.65 M Mg2+(aq) and 0.43 M PO43− (aq) in solution?
Answers
Answer:
115.6 kJ/mol
Explanation:
The ΔG of a reaction can be calculated using the following equation:
ΔG = ΔG° + RT ln(Q)
where:
ΔG° is the standard free energy change, which is given as 137.0 kJ/mol in this case
R is the gas constant, which is 8.314 J/(mol·K)
T is the temperature in Kelvin, which is 25°C + 273.15 = 298.15 K
Q is the reaction quotient, which is the ratio of the concentrations of the products to the concentrations of the reactants, each raised to their stoichiometric coefficients.
From the chemical equation given, the stoichiometric coefficients of Mg2+ and PO43- are 3 and 2 respectively. Therefore, the reaction quotient can be expressed as:
Q = [Mg2+]^3 [PO43-]^2
Substituting the given initial concentrations of Mg2+ and PO43- into the reaction quotient expression, we get:
Q = (0.65 M)^3 (0.43 M)^2 = 0.011 M^5
Now we can calculate the ΔG of the reaction:
ΔG = ΔG° + RT ln(Q)
ΔG = (137.0 kJ/mol) + (8.314 J/(mol·K) × 298.15 K) × ln(0.011 M^5)
ΔG = 137.0 kJ/mol - 21.38 kJ/mol
ΔG = 115.6 kJ/mol
Therefore, the ΔG for the reaction at 25°C and the given initial concentrations of Mg2+ and PO43- is 115.6 kJ/mol.
What molecule assists DNA in organization?
a.) Proteins
c.) Lipids
b.) Carbohydrates
d.) Salt
Answers
The molecules that assists deoxyribonucleic acid (DNA) in its organization is the proteins. That is option A.
What is Deoxyribonucleic acid (DNA)?Deoxyribonucleic acid (DNA) is defined as the hereditary molecules in a living organism that stores instructions for making other large molecules which are inherited from the parent.
In the structure of the deoxyribonucleic acid, they are made made up of base pairs that are organised by proteins known as histones.
Learn more about Deoxyribonucleic acid here:
https://brainly.com/question/28309571
#SPJ1
Phenolphthalein is used as an indicator for basic solutions in the electrolytic cell that's used to split water into its elements. Around which electrode would the solution turn pink?
Answers
Answer:
Anode
Explanation:
In the electrolysis of acidified water;
Anode half reaction;
4OH^-(aq)-----> 2H2O(l) + O2(g) + 4e
Cathode half reaction;
4H^+(aq) + 4e ----> 2H2(g)
Phenolphthalein has a pink colour in a neutral medium. A neutral medium in this electrolysis is one in which the hydroxide ion is reduced to water and oxygen and this occurs at the anode hence this is the electrode around which the solution will turn pink.
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pka for H2CO3 at body temperature is 6.1).
a) What is the pH of blood plasma?
b) If the volume of blood in a normal adult is 5.0L, what mass of HCl could be neutralized by the buffering system in blood before the pH falls below 7.0 (which would result in death)?
c) Given the volume from part (b), what mass of NaOH could be neutralized before the pH rises above 7.8?
Answers
Before the pH drops below 7.0, the buffering mechanism in blood can neutralise 1.26 kg of HCl.
(a) The equilibrium expression for the reaction between H₂CO₃ and HCO₃₋ is:
H₂CO₃ ↔ H+ + HCO₃₋
The pKa for H2CO3 at body temperature is 6.1, so the pH of blood plasma can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([HCO₃₋]/[H₂CO₃])
pH = 6.1 + log(0.024/0.0012)
pH = 7.4
Therefore, the pH of blood plasma is 7.4.
(b) The buffering capacity of blood can be calculated using the equation:
buffering capacity = Δ[HCO₃₋]/ΔpH
ΔpH is the change in pH that can be tolerated before the buffering system is overwhelmed. The buffering capacity of blood is highest at a pH of 7.4, which is the normal pH of blood plasma. Therefore, we will use a pH range of 7.4 ± 0.6 (i.e., from 6.8 to 8.0) to calculate the buffering capacity.
At pH 6.8, the [H+] is 10 times higher than at pH 7.4, so [H+] = 10^-6.8 = 1.58 × 10^-7 M.
At pH 8.0, the [H+] is 10 times lower than at pH 7.4, so [H+] = 10^-8.0 = 1.00 × 10^-8 M.
The change in [HCO3-] can be calculated using the Henderson-Hasselbalch equation:
[HCO₃₋] = [H₂CO₃]/(1 + 10^(pH-pKa))
At pH 6.8:
[HCO₃₋] = 0.0012/(1 + 10^(6.8-6.1)) = 0.0006 M
At pH 8.0:
[HCO₃₋] = 0.0012/(1 + 10^(8.0-6.1)) = 0.011 M
Δ[HCO₃₋] = 0.011 - 0.0006 = 0.0104 M
Therefore, the buffering capacity of blood is:
buffering capacity = 0.0104/0.6 = 0.0173 M/pH
The amount of HCl that can be neutralized before the pH falls below 7.0 can be calculated using the buffering capacity:
[H+] = 10⁻⁷ = 1.00 × 10⁻⁷ M
Δ[HCO₃₋] = buffering capacity × ΔpH = 0.0173 × (7.4 - 7.0) = 0.0069 M
The amount of HCl that can be neutralized is:
mass of HCl = Δ[HCO₃₋] × volume of blood × molar mass of HCl
mass of HCl = 0.0069 × 5.0 × 10^3 × 36.5 = 1.26 × 10³ g
Therefore, the buffering system in blood can neutralize 1.26 kg of HCl before the pH falls below 7.0.
To learn more about ph refer to:
brainly.com/question/15289741
#SPJ4
The sequence was already outlined, so she didn't have to make any difficult choices
to solve an equation
D) between math and poetry
to finish a task on time
D about which class to take
Answers
The sequences were already outlined therefore she didn't have any difficulty in choosing between math and poetry.
What is a sequence?A sequence is an orderly arrangement of events or objects. When objects or events are arranged in a sequence, decision making becomes much easier.
For the task at hand, since the sequences were already outlined, then she didn't have any difficulty in choosing between math and poetry.
Learn more about sequence: https://brainly.com/question/21961097
Answer:
to solve an equation
Which statements are true? Check all that apply.
All landforms have the same combinations of elevation and relief.
Different landforms have different combinations of elevation and relief.
Elevation is the height of a feature above sea level.
Relief is the height of a feature below sea level.
Relief is the difference between the highest and lowest points in an area.
Answers
The answer fam is.......
Different land forms have different combinations of elevation and relief.
Elevation is the height of a feature above sea level.
Relief is the difference between the highest and lowest points in an area.
The atomic number of an atom is
A. The mass of the atom.
B. The number of protons added to the number of neutrons in the nucleus.
C. The number of protons in the nucleus.
D. Negatively charged.
Answers
Answer:
B. the number of protons added to the number of neutrons in the nucleus.
Explanation:
Sana makatulong
Four processes are described below: Process A: Waves in a river lift rocks which crash down to the river bed and break Process B: Water in the cracks of rocks freezes and breaks open the rocks Process C: An organic acid produced by rotting vegetation makes holes in rocks Process D: Roots of plants push against rocks and make cracks in them Which process represents chemical weathering?
QUICKKKKKKKKKK ILL GIVE ALOT OF POINNTZS
Answers
Answer:
C: An organic acid produced by rotting vegetation makes holes in rocks Process
Explanation:
In chemical weathering , weathering process occurs due to chemical reaction . When organic acid produced by rotting vegetation makes holes in rocks , chemical reaction takes place between acid and rock which results in the formation of new product . Hence it is an example of chemical weathering .
In all the rest of options , weathering is due to physical process .
how many moles are in 6.7 x 10^25 molecules of H2SO4
Answers
Answer:
\( \huge{ \boxed{111.30 \: \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L} \\ }\)
where
n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities.
From the question.
N = 6.7 × 10²⁵ \( \: H_2SO_4 \: \) molecules
\(n = \frac{6.7 \times {10}^{25} }{6.02 \times {10}^{23} } \\ = 111.2956...\)
We have the final answer as.
111.30 moles
What is true of neutrons?
Answers
A powder contains feso47h2o
Answers
The mass of FeSO4*7H2O in the sample is 1.21 grams.
Calculate moles of Fe2O3
moles of Fe2O3 = mass of Fe2O3 / Molar mass of Fe2O3
moles of Fe2O3 = 0.348 grams / 159.69 g/mole = 0.00218 moles
Calculate moles of Fe
4 Fe + 3O2 → 2Fe2O3
For 4 moles of Fe consumed there is 2 moles of Fe2O3 produced
This means it has a ratio 2:1
So 0.00218 moles of Fe2O3 produced , there is 2*0.00218 = 0.00436 moles of Fe consumed
Calculate moles of FeSO4*7H2O
Fe + H2SO4 + 7H2O → FeSO4*7H20 + H2
For 1 mole of Fe consumed there is 1 mole of FeSO4*7H2O produced
This means for 0.00436 moles there is 0.00436 moles of Fe2SO4*H2O produced
Calculate the mass of FeSO4*7H2O in the sample
mass of FeSO4*7H2O = 0.00436 moles * 278.01 g/mole = 1.212 g
The mass of FeSO4*7H2O in the sample is 1.21 grams.
Complete question: A powder contains FeSO4⋅7H2O (molar mass=278.01 g/mol), among other components. A 3.930 g sample of the powder was dissolved in HNO3 and heated to convert all iron to Fe3+. The addition of NH3 precipitated Fe2O3⋅xH2O, which was subsequently ignited to produce 0.348 g Fe2O3. What was the mass of FeSO4⋅7H2O in the 3.930 g sample?
To learn more about Molar mass visit:https://brainly.com/question/12127540
#SPJ9
The volume of a sample of oxygen is 580.3 mL when
the pressure is 0.376 atm and the temperature is 43 °C.
At what temperature is the volume 907.1 L and the
pressure 0.415 atm?
a. 545
O b. 74.2
O c. -89.9
O d. 272
Answers
Answer:
545
Explanation:
If we let the unknown temperature in Kelvin be x,
\( \frac{0.376 \times 580.3}{43+273} = \frac{0.415 \times 907.1}{x} \\ \\ x = 545\)

The speed of Train A is 88 km/hr, and the speed of Train B is 96 km/hr. Based on this information, which of the following statements
is true?
ОА.
Train A has more kinetic energy than Train B.
OB. Neither train has any kinetic energy.
Ос.
The trains have the same kinetic energy.
OD
Train B has more kinetic energy than Train A
Answers
Answer:
C i took that test and got 96
Explanation:
Imagine that scientists have just discovered a non-bird dinosaur skeleton. They want to know whether the dinosaur was closely related to birds. What features in ...
might help them decide?
Answers
Answer:
This evidence includes fossilized bones, teeth, eggs, footprints, teeth marks, and even dung. When paleontologists compare a skeleton of a living bird to the fossilized skeleton of a non-bird theropod, like Sinornithosaurus, they see many similarities.
Explanation:
Answer:
When people think of dinosaurs, two types generally come to mind. There were the huge herbivores,
like Apatosaurus, with their small heads and long tails. There were also those fearsome carnivores,
like Tyrannosaurus rex, that walked on two legs and had a mouthful of teeth like kitchen knives.
Living Dinosaurs
These large dinosaurs are no longer around, but dinosaurs still live among us today. They are the
birds. It's difficult to imagine that a bird on your window sill and a T. rex have anything in common.
One weighs less than a pound. The other was the size of a school bus, tipping the scales at eight
tons. But for all their differences, the two are more similar than you might think. In fact, birds and T.
rex are close relatives. They all belong to a group of dinosaurs called theropods.
This is a cladogram, a "" showing the relationships among organisms. The group called dinosaurs includes the extinct dinosaurs
and all their living descendants. All its members, including living birds, descended from the very first dinosaur-their common ancestor.
That's why birds are a kind of dinosaur (just as humans are a kind of primate).
Skeletal Evidence
When paleontologists compare a skeleton of a living bird to the
fossilized skeleton of a non-bird theropod, like Sinornithosaurus,
they see many similarities. They both have a hole in the hipbone, a
feature that distinguishes most dinosaurs from all other animals.
This feature allows an animal to stand erect, with its legs directly
beneath its body. All theropod dinosaurs, including birds, have a
furcula, also known as a wishbone. Another shared characteristic is the presence of hollow bones.
Hollow bones reduce the weight carried by an animal. This feature enables the animal to run faster. It
probably also played a role in the evolution of flight.
thought to have evolved for flight. The discovery of more and more non-flying dinosaurs with feathers
disproved that explanation. For these dinosaurs, feathers may have served other functions, like
gliding, insulation, protection, and display. Feathers play that same role in many bird species today.
Based on the evidence of shared characteristics, scientists have concluded that birds are a type of
Birds are the only dinosaurs with the ability to fly. This is
very interesting to scientists who want to know when the
capability of flight emerged. To find out, some scientists
study the brains of bird and non-bird dinosaurs. Soft
tissue, such as brains, is almost never preserved in the
fossil record. What is preserved is the imprint the brain
left on the inside of the skull. Now scientists are using
computed tomography (CT) scanners to create
endocasts. These are detailed, three-dimensional
reconstructions of the interiors of fossilized skulls.
In a recent study, researchers were able to peer inside
the braincases of more than two dozen specimens.
"Technology allows us to look inside these specimens
without destroying them," says Dr. Amy Balanoff, a
Museum research associate. "It's a non-destructive way
to basically slice up a dinosaur brain. We look inside and see what it can tell us about the evolution of
the brain within dinosaurs. Most of us grew up thinking that dinosaurs had tiny brains, but actually
some had really big brains."
The endocasts allow Balanoff and other researchers to
explore the outer shape of the brain in more detail. In
addition, the casts also provide new information about
the volume and shape of different regions of the brain.
For example, scientists looked at a detailed view of the
dinosaur cerebrum, a region of the brain related to
cognition and coordination. They found that this region
was very large in non-bird dinosaurs closely related to
birds. Dr. Balanoff's research suggests that these
dinosaurs developed big brains long before flight and that
these bigger brains prepared the way for them to fly.
When examining skeletal, behavioral, and brain
evidence, scientists see that birds and non-bird dinosaurs
share many features. This helped them conclude that
dinosaurs aren't extinct after all. They're living among us today.
(Im a really fast Typer and Thinker)
Have a nice day
what is the change in mass of A in
60 minutes?
Mass of A (g)
12.4
10.4
9.1
7.7
6.2
Time
O
15
30
45
60
Answers
Answer:
To determine the change in mass of A over the given time period, we need to find the difference between the initial mass of A and the final mass of A.
From the given table, we can see that the initial mass of A at t = 0 (start time) is 12.4 g and the final mass of A at t = 60 minutes (end time) is 6.2 g.
Therefore, the change in mass of A over 60 minutes is:
Final mass of A - Initial mass of A
= 6.2 g - 12.4 g
= -6.2 g
The negative sign indicates that the mass of A decreased over time, which means that A underwent some kind of reaction or process that caused it to lose mass.
The change in mass of A over 60 minutes is -6.2 grams.
To determine the change in mass of A over 60 minutes, we need to compare the initial mass to the final mass.
From the given information, we can see that the mass of A decreases over time.
Let's calculate the change in mass.
Initial Mass of A: 12.4 g
Final Mass of A: 6.2 g
Change in Mass of A = Final Mass of A - Initial Mass of A
= 6.2 g - 12.4 g
= -6.2 g
The change in mass of A over 60 minutes is -6.2 grams.
Note that the negative sign indicates a decrease in mass.
For such more questions on mass
https://brainly.com/question/1838164
#SPJ8
what does the term normal imply when used in Alkanes?
Answers
Answer:
The simplest alkanes have their C atoms bonded in a straight chain; these are called normal alkanes. They are named according to the number of C atoms in the chain.
A local orchard sells bags of red apples by the dozen. The packaging
department of the orchard determines the mass of each dozen batch of
red apples before bagging them. The bag is then labeled with the mass of
the apples. Observe the mass of the dozen red apples shown on the scale.
Based upon this mass, what would the mass of 7 red apples be in
kilograms? Assume that each of the dozen apples on the scale has the
same mass. Answer is rounded to one place after the decimal. 0.5 kg
2.00 kg

Answers
Answer:
2.0kg
Explanation:
The mass of 7 red apples in kilograms is to be considered as the 1.16 kilograms.
Calculation of the mass:Since the mass of a dozen apples is 2 kg.
we know that
1 dozen is 12 units
So the mass of 12 apples = 2kg
So mass of 1 apple = 2/12 = 1/6 kg
Now the mass of 7 apple is to be
= 7/6 kg
= 1.16 kg
hence, The mass of 7 red apples in kilograms is to be considered as the 1.16 kilograms.
Learn more about mass here: https://brainly.com/question/24701836
The Sun has been shining on this swimming pool all day. The water is much warmer than it was in the morning. Describe what is happening to the water in terms of temperature, particle speed, and kinetic energy.
Answers
Answer:
The waters' temp increased
Explanation:
The temperature of the water in the swimming pool has increased due to the heat from the Sun. As a result, the particles in the water are moving faster and have a higher kinetic energy than in the morning.
how do i convert 145.6grams of Iron sulphide into iron and sulfur
Answers
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8