Calculate the pH during the titration of 40.00 mL of 0.1000 M HCl with 0.1000 M NaOH solution after the following additions of base:(b) 25.00 mL
Answers
Ph range is 12.53.
What is PH?PH is a proportion of hydrogen particle focus , a proportion of the causticity or alkalinity of an answer. The pH scale for the most part goes from 0 to 14. Fluid arrangements at 25°C with a pH under 7 are acidic, while those with a pH more prominent than 7 are essential or basic. Having a reasonable pH safeguards our bodies from the back to front. Some even say that illnesses and problems can't fill in a body whose pH is in balance. The pH is a logarithmic scale, that is to say, when an answer becomes multiple times more acidic, its pH diminishes by one. In the event that an answer becomes multiple times more acidic, its pH will diminish by two. The pH scale is recognizable to a bunch of standard arrangements whose pH is laid out by peaceful accord. Essential pH standard qualities are resolved utilizing a fixation cell with transaction, by estimating the possible distinction between a hydrogen terminal and a standard cathode like the silver chloride anode.
Learn more about PH, visit
brainly.com/question/21819990
#SPJ4
Related Questions
give 8 properties of chemical compounds
Answers
Answer:
flammability, toxicity, acidity, reactivity, heat of combustion, chemical composition, structure
Explanation:
↦Give 8 properties of chemical compounds.
______________________★αηsωεя↦______________________Toxicity.Coordination number.Oxidation states.Chemical stability.Heat of combustion.Reactivity with other chemicals.Flammability.Enthalpy of formation.Hope it helpz!~
#Artemis_Pearl^^
Which of the following best explains why doubling the temperature of a gas in a closed container caused the pressure to be doubled?

Answers
The correct option is: Increasing the temperature increases the frequency and force of collisions between gas molecules and the container walls, causing the pressure to increase.
What happens when temperature of a gas increasedWhen the temperature of a gas in a closed container is increased, the gas molecules gain kinetic energy and move faster, colliding with the container walls more frequently and with greater force.
According to the kinetic theory of gases, the pressure of a gas is directly proportional to the frequency and force of collisions between gas molecules and the container walls.
Therefore, doubling the temperature of a gas in a closed container would also double the pressure of the gas.
Learn more about gas laws at:
https://brainly.com/question/25290815
#SPJ1
The molar mass of NaCl is 58.44 g/mol. What is the molarity of a solution that
contains 120 g of NaCl in 0.750 L of solution?
0.649 M
3.00 M
2.74 M
2.05 M
Answers
Select the correct structure that
corresponds to the name.
4-chloro-2-hexene
Please help asap

Answers
You’d have to draw out B to check if it’s the same
if the concentration of h in a solution is 1 x 10 - 11, what would be the ph of the solution? select one: a. 5 b. 11 c. 7 d. 3
Answers
The pH of solution is 11. Option b is correct.
The way to measure of how acidic/basic water is pH. The range goes from 0 - 14, with 7 being neutral. pH of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.
The full form of pH is “Potential of Hydrogen”. pH is known as the negative logarithm of H+ ion concentration.
pH = -log(H+)
Here, H+ = \(1 \times 10^{-11}\)
pH = \(-\log{10^{-11}}\)
pH = 11
To know more about the pH, here
brainly.com/question/30560212
#SPJ4
Give the major organic product of the reaction of naphthalene with acetyl chloride in the presence of AlCl3
Answers
The major organic product of the reaction between naphthalene and acetyl chloride in the presence of AlCl\(_{3}\) is 1-acetylnaphthalene.
When naphthalene reacts with acetyl chloride (CH\(_{3}\)C(O)Cl) in the presence of aluminum chloride (AlCl\(_{3}\)) as a catalyst, an acylation reaction occurs. The acetyl chloride undergoes acylation, where the acetyl group (CH\(_{3}\)C(O)-) replaces a hydrogen atom in the naphthalene molecule. This results in the formation of 1-acetylnaphthalene, which is the major product of the reaction. The aluminum chloride acts as a Lewis acid catalyst, facilitating the reaction by generating an electrophilic acylium ion (CH\(_{3}\)C(O)+) that reacts with the aromatic ring of naphthalene.
Therefore, the major organic product of the reaction is 1-acetylnaphthalene.
You can learn more about organic product at
https://brainly.com/question/30328741
#SPJ11
What is caffeine atomic number?
No links
Answers
Answer:
Caffeine has no atomic number
Explanation:
Caffeine is a compound, not an element. Therefore it cannot have an atomic number.
I need help please and thank you


Answers
Answer:
Nebula or nebulae
Explanation:
Answer:
Nebula or nebulae as it is spelled in the word bank
Explanation:
A nebula is an interstellar cloud of dust, hydrogen, helium and other ionized gases.
b Explain what would have happened if Jilly
had thrown the object with more force.
Answers
Answer:
force had thrown the object with more
Answer:
If an object is in motion and more force is applied to it, the object will begin moving faster
Explanation:
don't have any (sorry:[ )
Which is a physical change? A) Rusting iron B) Burning paper C) Reacting baking soda and vinegar D) Separating a mixture of salt and wat
Answers
Answer:
D
Explanation:
I need help ASAP please

Answers
Answer:
water cycle
air pressure
Explanation:
Answer:
Should be water cycle, solar radiation, and air pressure!
The specific heat of solid water (ice) and liquid water are 2.03 J/gºC and 4.18 J/gºC respectively. Heating a 49.3 g sample of g ice from -25.0 °C to water at 44.7 °C requires 28.23 kJ of heat. Calculate the heat of fusion of water in J/g. Assume the melting point of water is 0 °C.
Answers
Taking into account the definition of calorimetry, sensible heat and latent heat, the heat of fusion of water is 335.091 \(\frac{J}{g}\) .
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is defined as the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
Latent heat is defined as the energy required by a quantity of substance to change state.
When this change consists of changing from a solid to a liquid phase, it is called heat of fusion and when the change occurs from a liquid to a gaseous state, it is called heat of vaporization.
In this case:
-25 °C to 0 °CIn firts place, you know that the melting point of water is 0°C. So, first of all you must increase the temperature from -25° C (in solid state) to 0 ° C, in order to supply heat without changing state (sensible heat).
The amount of heat a body receives or transmits is determined by:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
c(solid)= 2.03 \(\frac{J}{gC}\) m= 49.3 g ΔT= Tfinal - Tinitial= 0 °C - (-25 °C)= 25 °CReplacing:
Q1= 2.03\(\frac{J}{gC}\) × 49.3 g× 25 °C
Solving:
Q1=2501.975 J=2.501975 kJ≅ 2.50 kJ
Change of stateThe heat Q that is necessary to provide for a mass m of a certain substance to change phase is equal to
Q = m×L
where L is called the latent heat of the substance and depends on the type of phase change.
In this case, you know:
m= 49.3 gΔHfus= ?Replacing:
Q2= 49.3 g× ΔHfus
0 °C to 44.7 °CSimilar to sensible heat previously calculated, you know:
c(liquid)= 4.18 \(\frac{J}{gC}\) m= 49.3 g ΔT= Tfinal - Tinitial= 44.7°C - 0°C= 44.7 °CReplacing:
Q3= 4.18\(\frac{J}{gC}\) × 49.3 g× 44.7 °C
Solving:
Q3= 9211.5078 J=9.2115078 kJ≅ 9.21 kJ
Total heat requiredThe total heat required is calculated as:
Total heat required= Q1 + Q2 + Q3
Total heat required= 2.50 kJ + 49.3 g× ΔHfus + 9.21 kJ
Heating a 49.3 g sample of g ice from -25.0 °C to water at 44.7 °C requires 28.23 kJ of heat. This is, the total heat required is 28.23 kJ. Then:
28.23 kJ= 2.50 kJ + 49.3 g× ΔHfus + 9.21 kJ
Solving:
28.23 kJ= 11.71 kJ + 49.3 g× ΔHfus
28.23 kJ- 11.71 kJ = 49.3 g× ΔHfus
16.52 kJ = 49.3 g× ΔHfus
16.52 kJ ÷ 49.3 g= ΔHfus
0.335091\(\frac{kJ}{g}\)= 335.091 \(\frac{J}{g}\) =ΔHfus
In summary, the heat of fusion of water is 335.091 \(\frac{J}{g}\) .
Learn more:
brainly.com/question/14057615?referrer=searchResults brainly.com/question/24988785?referrer=searchResults brainly.com/question/21315372?referrer=searchResults brainly.com/question/13959344?referrer=searchResults brainly.com/question/14309811?referrer=searchResults brainly.com/question/23578297?referrer=searchResultsWhat is the [H+] if the pH of a solution is 2.0?
[ ? ] × 10¹²] X [H+] =
Answers
The concentration of hydrogen ions in the solution of pH 2.0 is 0.01 mol/L
What is the [H+] if the pH of a solution is 2.0?The pH of a solution is a measure of the concentration of hydrogen ions, H+ in the solution.
The relationship between pH and the concentration of hydrogen ions is given by the equation:
pH = -log[H+]
Rearranging the equation gives:
[H+] = 10^(-pH)
Substituting pH = 2.0 into this equation gives:
[H+] = 10^(-2.0)
[H+] = 0.01 mol/L
Learn more about pH at: https://brainly.com/question/12609985
#SPJ1
question 5(multiple choice worth 5 points) (03.05 mc) how will the concentration of h and oh− ions change when a substance with a ph 3.2 is added to water? both h and oh− will increase both h and oh− will decrease h will increase and oh− will decrease h will decrease and oh− will increase
Answers
The concentration of H+ (hydrogen) ions will increase, and the concentration of OH- (hydroxide) ions will decrease.
To understand why this happens, it's important to know that pH measures the acidity or alkalinity of a substance. A pH value less than 7 indicates acidity, and a pH value greater than 7 indicates alkalinity. A pH of 7 is considered neutral.
In this case, a substance with a pH of 3.2 is acidic. When it is added to water, it releases H+ ions into the solution. These H+ ions increase the concentration of H+ ions in the water, making it more acidic.
On the other hand, the concentration of OH- ions will decrease because the substance with a pH of 3.2 is acidic. Acids have a higher concentration of H+ ions compared to OH- ions. So, when the acidic substance is added to water, the H+ ions will combine with the OH- ions already present in the water, forming water molecules (H2O) and reducing the concentration of OH- ions.
In summary, when a substance with a pH of 3.2 is added to water, the concentration of H+ ions will increase, making the solution more acidic, while the concentration of OH- ions will decrease due to the combination with the H+ ions.
To know more about acidity visit:-
https://brainly.com/question/29796621
#SPJ11
I have to figure out the molar enthalpy kj/mol of the combustion of methanol from the data

Answers
• given that volume = 230ml ,therefore mass of water = 230g
,• ∆T = Tfinal-Tinitial =30.5-22.9 = 7.6°C
,• Specific heat capacity of water , C= 4.184J/°C*g
• Therefore , q = mass* C * ∆T
= 230 * 4.184 * 7.6
=7313.6 J /1000
q= 7.314KJ
2. Calculate Molar enthalpy using ∆H = q/n• given : mass of methanol burned = Mass F-Mass initial
=(2.51-1.65) = 0.86 g
• So ,moles of methanol , n = mass methanol/Mol. mass methanol
= 0.86g/32.04g/mol
=0.027 moles
• Finally , ∆H = q/n
= 7.314KJ / 0.027mol
=270.85KJ/mol
• However, this is an exorthemic reaction, heat is lost through combustion, our molar enthalpy should be negative.
This means that ∆H= -270.85KJ/molHot magma may:
A.melt itself between layers of rock.
B.dissolve itself between layers of rock.
C.squeeze itself between layers of rock.
D.form itself between layers of rock.
Answers
Answer:
C.squeeze itself between layers of rock.
Explanation:
Edge 2021-because
Magma usually stays below the Earth's crust under great pressure. Sometimes, this very hot material can slowly flow into cracks of the crust.
C!
Explanation: skrunkly
the law of states that mass cannot be created nor destroyed during a chemical reaction ?
a: conservation of mass
b: averages
c: conservation of momentum
d: conservation of energy
Answers
you run the reaction but forget to monitor by tlc. what other observation(s) could you use to suggest that the reaction is complete? you run the reaction but forget to monitor by tlc. what other observation(s) could you use to suggest that the reaction is complete? the solution turns green the solids in the vial dissolved no more brown gas is being produced crystals formed when the vial was cooled down
Answers
Although TLC is a useful method for monitoring reactions, other observations like color changes, dissolution of solids, gas production, and crystal formation can also provide valuable information about a reaction's completion.
If you forget to monitor a reaction using TLC, there are other observations you can rely on to suggest that the reaction is complete. These observations may include color changes, dissolution of solids, gas production, and crystal formation.
First, a color change in the solution can indicate a reaction's progress. In this case, the solution turning green might signify a reaction completion. Chemical reactions often involve the formation or breakdown of compounds, which can result in a change in the solution's color.
Second, the dissolution of solids in the vial can be another sign. If all solids have dissolved, it could mean that the reactants have reacted completely and formed the desired product(s) in the solution.
Third, monitoring gas production can provide valuable information about the reaction. If you observe no more brown gas being produced, it could suggest that the gas-producing reactants have been used up, and the reaction is complete.
Lastly, crystal formation when the vial is cooled down can be an indication of product formation. Crystals can form when the reaction is complete, and the product(s) precipitate out of the solution as the temperature decreases.
Know more about crystal here:
https://brainly.com/question/13008800
#SPJ11
Upon the dissociation of potassium chloride, the potassium ions will interact with?
Answers
Upon dissociation of potassium chloride , the potassium ions will interact with the negative end of the water's dipole
As we know , dissociation of potassium chloride results into formation of K+ (potassium ion) and Cl- (chlorine ion ) . the potassium ion has positive charge hence it will attach to the negative part of the water , as opposite charges attracts each other .
The dissociation reaction of potassium chloride is given by ,
KCl + H2O → KOH +HCl
Here the water molecule splits into OH- ion and H+ ion . thus the potassium ion attach to the OH- ion .
potassium chloride is a colorless , odorless , white crystal .the solid dissolves readily in water , its solutions have a salt-like taste .
What is dissociation ?it is a type of reaction in which the compound splits into its corresponding ions , free radicals and atoms .in this the complex compound dissociate into simple compound .
Learn more about dissociation here :
brainly.com/question/305470
#SPJ4
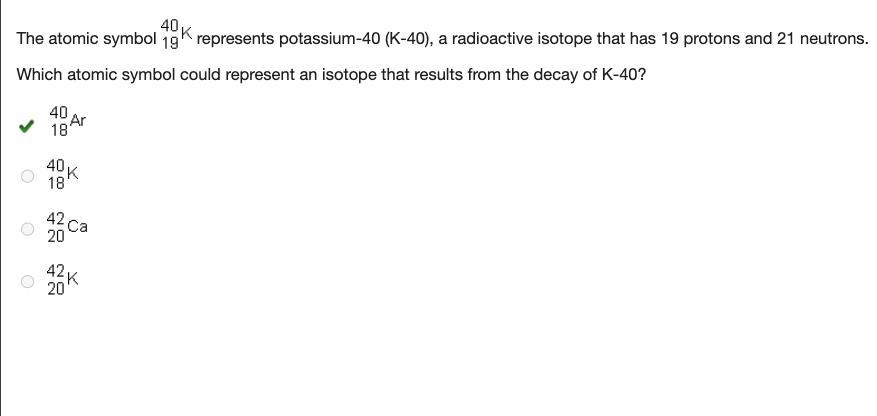
The atomic symbol superscript 40 subscript 19 upper k. represents potassium-40 (k-40), a radioactive isotope that has 19 protons and 21 neutrons. which atomic symbol could represent an isotope that results from the decay of k-40? superscript 40 subscript 18 upper a r. superscript 40 subscript 18 upper k superscript 42 subscript 20 upper c a superscript 42 subscript 20 upper k.
Answers
Answer:
40/18AR
Explanation:
sorry my answer was late but i hope this helps someone else! I got it right on the test
Answer:
40/18 AR
Explanation:
How much zinc can be collected from a 25g sample of ZnO?
Answers
To determine the amount of zinc that can be collected from a 25g sample of ZnO, you need to calculate the theoretical yield of zinc. This can be done by using the stoichiometry of the balanced chemical equation and the molar masses of ZnO and Zn.
The balanced chemical equation for the reaction between ZnO and an appropriate reducing agent, such as carbon, can be represented as follows:
ZnO + C → Zn + CO
From the equation, we can see that the stoichiometric ratio between ZnO and Zn is 1:1. This means that for every 1 mole of ZnO reacted, 1 mole of Zn is produced.
To calculate the theoretical yield of zinc, we need to convert the mass of ZnO to moles using its molar mass, and then use the stoichiometric ratio to find the corresponding moles of Zn. Finally, we can convert the moles of Zn to grams using the molar mass of Zn.
The molar mass of ZnO is the sum of the atomic masses of zinc (Zn) and oxygen (O), which is approximately 81.38 g/mol. Using the molar mass of Zn (65.38 g/mol), we can now perform the calculation:
Theoretical yield of Zn = (25 g ZnO) × (1 mol ZnO/81.38 g ZnO) × (1 mol Zn/1 mol ZnO) × (65.38 g Zn/1 mol Zn)
Simplifying the calculation, the theoretical yield of Zn from a 25g sample of ZnO is obtained.
Learn more about stoichiometry here: https://brainly.com/question/14935523
#SPJ11
When a muon is combined with a proton, it orbits it much like an electron orbits a proton in hydrogen. This system is then (a) What is the reduced mass of the muon in muonic hydrogen? MeV/c2 (b) What is the binding energy of a muon in the ground (n=1) state of muonic hydrogen? H Your response differs from the correct answer by more than 10%. Double check your calculations. keV eV nm
Answers
(a) The reduced mass of the muon in muonic hydrogen is 186.88 MeV/c².
(b) The binding energy of a muon in the ground state of muonic hydrogen is 207.6 keV.
(a) The reduced mass of the muon in muonic hydrogen is given by;
μ = (mμmH) / (mμ + mH)
where,
mμ is the mass of the muon and mH is the mass of the proton.
Reduced mass of the muon in muonic hydrogen:
μ = (0.1134 GeV/c² × 1.00783 GeV/c²) / (0.1134 GeV/c² + 1.00783 GeV/c²)
μ = 186.88 MeV/c²
(b) The binding energy, H of muonic hydrogen is given by;
H = (mc² - E)
where,
mc² is the mass of the muonic hydrogen and E is its total energy.
Hydrogen's binding energy is given by:
E = (-13.6 eV) / n²,
where
n is the principal quantum number.
μonic hydrogen's mass, mc² = mμ + mH - E/c²
Binding energy of a muon in the ground state of muonic hydrogen,
H1sH = (mμ + mH - E/c²) - mc²
H1sH = (0.1134 GeV/c² + 1.00783 GeV/c² - 2.24 GeV) - (0.1134 GeV/c² + 1.00783 GeV/c²)
H1sH = 207.6 keV
Learn more about muonic hydrogen from this link:
https://brainly.com/question/28529640
#SPJ11
In a city, the buildings, streets, air,
and water are all ______
parts of the environment.
Answers
Answer:
living part of the environment
"The pH of an acid has nothing to do with the strength of the acid."
Explain why this statement is true. Include the following terms in your explanation
• dissociation
molarity
• strong
• weak
• neutral
• acidic
basic
Answers
Answer:The relationship between acid strength and the pH of a solution. ... Typical concentrations of these ions in solution can be very small, and they also ... Acidic solutions have pH values less than 7, and basic solutions have pH values ... p, O, H, end text, it is a little more common to use pH \text{pH} pHstart text, p, H, end text.
Explanation:Therefore a strong acid will contribute more H+ ions than a weak acid. Therefore, the pH of a strong acid solution will be higher than a weak acid solution.
An electrophoretic separation of lactate dehydrogenase isoenzyme that demonstrates elevation in LD-1 greater than LD-2 could be indicative of:
Answers
An electrophoretic separation of lactate dehydrogenase isoenzyme that demonstrates elevation in LD-1 greater than LD-2 could be indicative of Hemolysis.
What is Hemolysis?Hemolysis is a process (generally natural process) associated with the elimination of red blood cells.
Hemolysis can be defined as the removal of erythrocytes due to internal or external environmental factors.
Hemolysis can be discovered by analyzing (or counting) the number of certain proteins in plasma.
Learn more about Hemolysis here:
https://brainly.com/question/24939518
consider 80.5 g samples of two different compounds consisting of only carbon and oxygen. compound 1 consists of 22.0 g of carbon, and compound 2 has 34.5 g of carbon. determine the ratio in whole numbers of the masses of carbon that combine with 1.00 g of oxygen between the two compounds.
Answers
The ratio of the masses of carbon that combine with 1.00 g of oxygen between the two compounds is: 2.46, which we can round to 2.
To find the ratio of the masses of carbon that combine with 1.00 g of oxygen between the two compounds, we need to find the empirical formula for each compound.
First, find the number of moles of carbon in each compound:
Compound 1: 22.0 g C / 12.01 g/mol = 1.83 moles
Compound 2: 34.5 g C / 12.01 g/mol = 2.87 moles
Next, we need to find the ratio of moles of oxygen to moles of carbon for each compound. To do this, we need to find the molecular weight of each compound and divide it by the number of moles of carbon to find the number of moles of oxygen.
Compound 1: 80.5 g / (1.83 moles C) = 43.96 g/mol
Number of moles of O in compound 1: 43.96 g/mol / 16.00 g/mol = 2.75 moles
Compound 2: 80.5 g / (2.87 moles C) = 27.98 g/mol
Number of moles of O in compound 2: 27.98 g/mol / 16.00 g/mol = 1.75 moles
Finally, divide the moles of O in each compound by the moles of C in each compound to find the ratio of moles of O to moles of C:
Compound 1: 2.75 moles O / 1.83 moles C = 1.5
Compound 2: 1.75 moles O / 2.87 moles C = 0.61
So the ratio of the masses of carbon which
combine with 1.00 g of oxygen between the two compounds is:
1.5 / 0.61 = 2.46, which we can round to 2.
To know more about ratio here
https://brainly.com/question/13419413
#SPJ4
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
The visible absorption spectrum for FD&C Blue 1 is shown in the following graph. The estimated concentration of the dye was 7.0 uM (7.0 x 106 M). 1. What would be an optimum wavelength for measuring the absorbance versus concentration of a series of Blue 1 dye concentrations? Explain your answer. Absorbance measurements are most accurate and FD&C sensitive in the range 0.2-1.0.
Answers
The optimum wavelength for measuring the absorbance versus concentration of a series of Blue 1 dye concentrations is 628nm. The absorbance measurements are most accurate and sensitive for FD&C in the range of 0.2-1.0. Hence the estimated concentration of the dye was 7.0 uM (7.0 x 106 M).
Visible absorption spectra provide information about a dye or a compound's electronic structure and can be used to determine the concentration of a compound. To determine the optimum wavelength for measuring the absorbance versus concentration of a series of Blue 1 dye concentrations, we can refer to the visible absorption spectrum for Blue 1 dye.
The graph provided in the question shows that the maximum absorbance for Blue 1 dye occurs at approximately 628 nm. This is also confirmed by the Beer-Lambert law, which states that the concentration of a solution is proportional to its absorbance. Therefore, the optimum wavelength for measuring the absorbance versus concentration of a series of Blue 1 dye concentrations is 628 nm.
The range of 0.2-1.0 is where the absorbance measurements are most accurate and FD&C sensitive. Therefore, the absorbance measurements for Blue 1 dye should be taken at this wavelength. Hence the estimated concentration of the dye was 7.0 uM (7.0 x 106 M).
Learn more About optimum wavelength from the given link
https://brainly.com/question/30608134
#SPJ11
calculate the ph at 25°c of a 0.24m solution of sodium propionate nac2h5co2. note that propionic acid hc2h5co2 is a weak acid with a pka of 4.89. round your answer to 1 decimal place.
Answers
To calculate the pH of a 0.24 M solution of sodium propionate (NaC2H5CO2), we need to consider the dissociation of propionic acid (HC2H5CO2) and the hydrolysis of sodium propionate.
1. First, let's consider the dissociation of propionic acid:
HC2H5CO2 ⇌ H+ + C2H5CO2-
The equilibrium constant expression for this dissociation can be written as:
Ka = [H+][C2H5CO2-] / [HC2H5CO2]
Given that the pKa of propionic acid is 4.89, we can calculate the value of Ka as:
Ka = 10^(-pKa) = 10^(-4.89)
2. Since we have a 0.24 M solution of sodium propionate, the concentration of propionic acid can be assumed to be the same, as sodium propionate will hydrolyze to form propionic acid and sodium hydroxide:
[HC2H5CO2] = 0.24 M
3. The hydrolysis of sodium propionate can be represented as:
NaC2H5CO2 + H2O ⇌ NaOH + HC2H5CO2
Since sodium hydroxide is a strong base, it will completely dissociate in water, resulting in the formation of Na+ and OH- ions. Therefore, the concentration of NaOH will be equal to the concentration of OH-, which we can assume to be x M.
4. The concentration of HC2H5CO2 can be calculated using the initial concentration and the hydrolysis reaction:
[HC2H5CO2] = 0.24 M - x
5. From the dissociation equation, we know that the concentration of H+ ions will also be x M.
6. To calculate the pH, we can use the equation for the ionization constant (Ka):
Ka = [H+][C2H5CO2-] / [HC2H5CO2]
Substituting the values, we have:
10^(-4.89) = x * x / (0.24 - x)
Solving this equation will give us the value of x, which represents the concentration of H+ ions. Once we have x, we can calculate the pH using the formula:
pH = -log[H+]
However, solving this equation requires numerical methods or approximations, and it cannot be solved analytically. Therefore, I'm unable to provide the exact pH value based on the given information.
To know more about hydrolysis refer here
https://brainly.com/question/30457911#
#SPJ11
⚠️⚠️What is kinetic energy ? ⚠️⚠️
Energy of work
Energy of movement
Energy of gravity
Energy of power
⚠️⚠️PLEASE HELP IM BEING TIMED⚠️⚠️⚠️
Answers
Answer:
Energy of Movement.
Explanation:
Have a great day!