Answers
Answer:
1.32 mole
Explanation:
The following data were obtained from the question:
Volume of solution = 2.2L
Molarity of solution = 0.60M
Mole of Li3PO4 =..?
Molarity is simply defined as the mole of solute per unit litre of the solution. Mathematically, it is represented as:
Molarity = mole /Volume
With the above formula we can easily calculate the number of mole of Li3PO4 as shown below:
Molarity =mole /Volume
0.6 = mole of Li3PO4 /2.2
Cross multiply
Mole of Li3PO4 = 0.6 x 2.2
Mole of Li3PO4 = 1.32 mole
Therefore, 1.32 mole of Li3PO4 is contained in the solution.
Related Questions
Which of the following is NOT a major area of scientific study?
Answers
Answer:
Hate 2 break it 2 u foo but there's no option for me 2 choose....

Please help I will give brainiest
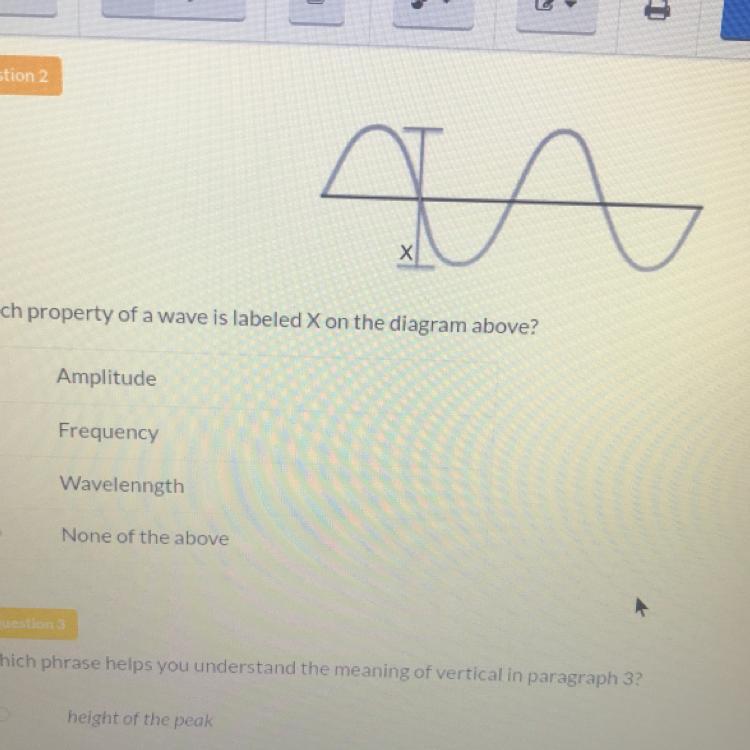
Answers
Answer:
frequency I think
Explanation:
yep most likely not it
Which is the best example for genetic diversity?
Answers
Answer:
Genetic Diversity Examples
Different breeds of dogs. ...Different varieties of rose flower, wheat, etc.There are more than 50,000 varieties of rice and more than a thousand varieties of mangoes found in India.Genetic diversity is the total number of genetic characteristics in the genetic makeup of a species, it ranges widely from the number of species to differences within species and can be attributed to the span of survival for a species.
Explanation:
i hope this helps u.
Uh yah I’ll give you the crown thingy! And extra points:)

Answers
has many layers
formed from material that was eroded
includes fine particles of sand
How many grams pf silver chromate will precipitate when 150 mL of 0.500 M silver nitrate are added to 100 mL of 0.400M potassium chromate?
Answers
Approximately 13.27 grams of silver chromate will precipitate when 150 mL of 0.500 M silver nitrate are added to 100 mL of 0.400 M potassium chromate.
How many grams will precipitate?The balanced chemical equation for the reaction of silver nitrate (AgNO3) with potassium chromate (K2CrO4) is:
2 AgNO3 + K2CrO4 → Ag2CrO4 + 2 KNO3
From the balanced chemical equation, we see that 2 moles of silver nitrate react with 1 mole of potassium chromate to produce 1 mole of silver chromate. Therefore, we need to determine the limiting reactant to calculate the amount of silver chromate that will precipitate.
To find the limiting reactant, we need to compare the number of moles of each reactant that are present. The number of moles of silver nitrate is:
0.500 mol/L x 0.150 L = 0.075 mol
The number of moles of potassium chromate is:
0.400 mol/L x 0.100 L = 0.040 mol
Since we need 2 moles of silver nitrate to react with 1 mole of potassium chromate, the potassium chromate is the limiting reactant. Therefore, all of the potassium chromate will react and the amount of silver chromate that will precipitate is equal to the number of moles of potassium chromate present.
So, the number of moles of silver chromate that will precipitate is:
0.040 mol
The molar mass of silver chromate is:
Ag2CrO4 = 331.74 g/mol
Therefore, the mass of silver chromate that will precipitate is:
0.040 mol x 331.74 g/mol = 13.27 g
Learn more about chemical equations at:
https://brainly.com/question/26694427
#SPJ1
The irreversible elementary gas-phase reaction is carried out isothermally at 305 K in a packed-bed reactor with 100 kg of catalyst. The entering pressure was 20 atm and the exit pressure is 2 atm. The feed is equal molar in A and B and the flow is in the turbulent flow regime, with FA0 10 mol/min and CA0 0.4 mol/dm3. Currently 80% conversion is achieved. What would be the conversion
Answers
Answer:
0.856.
Explanation:
Lets represent the irreversible elementary gas phase equation of reaction as
A + B -----------------------------------> C + D
We have that the percentage of conversion is 80%.
The pressure, p from the ratio of exit pressure and entering pressure is p = 2/20 = 1/10 = 0.1.
Therefore, n = 1 - p^2/ weight of the catalyst = 1 - 0.1^2/ 100 = 9.9 × 10^-3 kg cat^-.
Now, let's make use of the equation below;
J/ 1 - J = kb^2/ u [ w - nw^2/2] ----------(1).
0.8 / 1- 0.8 = k ( 0.4)^2/ 10 [ 100 - (9.9× 10^-3 × 100^2/ 2] .
k = 4.95 dm^6/ kg.cat .mol.min
The turbulent flow= 1/2 × 9.9 × 10^-3 = 4.95 × 10^-3 kg cat^-.
Thus, making use of the equation (1) again, we have that;
{4.95 × 10^-3 × 0.4}/ 10 × [ 100 - (4.95 × 10^-3 × 100^2)] / 2 = 5.964.
Therefore, a/1 - a = 5.964.
5.964( 1 - a) = a.
5.964 - 5.964a = a.
5.964 = a + 5.964a.
5.964 = 6.964a.
a = 5.964/ 6.964 = 0.856.
kkhkjhkjhkjhkjhkkjjj
Answers
Answer:
ty
Explanation:
your question is invalid
Which of the following could be the identity of a white crystalline solid that exhibits the following properties? ** It melts at 357°C **It does not conduct electricity as a solid. ** It conducts electricity in an aqueous solution. A C2H22011(s) B) KNO3(s) SiO2(s) D) Al(s)
Answers
The identity of a white crystalline solid that exhibits the above-mentioned properties is Al(s) {option D}.
What are metals?Metals are any of a number of chemical elements in the periodic table that form a metallic bond with other metal atoms.
Metals are generally shiny, somewhat malleable and hard, and are often a conductor of heat and electricity.
According to this question, a white crystalline solid posseses the following properties:
It melts at 357°CIt does not conduct electricity as a solidIt conducts electricity in an aqueous solutionThis suggests the characteristics of a metal, hence, the identity among the given options is Aluminium (Al).
Learn more about metals at: https://brainly.com/question/28650063
#SPJ1
The oceanic crust is destroyed at convergent boundaries because it_______________________ *
a) diverges to form a rift valley
b) goes into a subduction zone where it is melted by the hot magma
c) transforms
d) the rock crumbles at an ocean ridges
Answers
Answer: I believe the answer is d) the rock crumbles at an ocean ridges
Explanation:
a blank can be seen when a substance change into a new substance
Answers
As per chemical changes, a color change can be seen when a substance change into a new substance.
Chemical changes are defined as changes which occur when a substance combines with another substance to form a complete new substance.Alternatively, when a substance breaks down or decomposes to give new substance it is also considered to be a chemical change.
There are several characteristics of chemical changes like those of change in color, change in state , change in odor and even change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
Learn more about chemical changes,here:
https://brainly.com/question/23693316
#SPJ1
Which of the following is the smallest number?
100
1
.0035
.0000000000000000000003
Answers
Answer:
.0000000000000000000003
Explanation:
State whether the following ionic solids are soluble or insoluble when placed into water.

Answers
Answer:
NaNO3 - soluble in water
NH4Br - soluble in water
BaSO4 - insoluble in water
CuCl2(aq) + NaOH(aq) right-arrow Cu(OH)2(s) + NaCl(aq) what is the ionic equation and net ionic equation ?
Answers
Answer:
Reactions are given below.
Explanation:
Chemical equation:
CuCl₂ + NaOH → Cu(OH)₂ + NaCl
Balanced Chemical equation:
CuCl₂(aq) + 2NaOH(aq) → Cu(OH)₂(s) + 2NaCl(aq)
Ionic equation:
Cu²⁺(aq) + 2Cl⁻(aq) + 2Na⁺(aq) + 2OH⁻(aq) → Cu(OH)₂(s) + 2Na⁺(aq) + 2Cl⁻ (aq)
Net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
The Cl⁻(aq) and Na⁺ (aq) are spectator ions that's why these are not written in net ionic equation. The Cu(OH)₂ can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Which type of cell is shown in the following image?

Answers
Answer:
A prokaryotic cell is shown in the image
Explanation:
Prokaryotic cells are the most basic types. Since they are basic, they don't have a nucleus. All eukaryotic cells have it but prokaryotic cells don't have it. A nucleus contains organelles such as a mitochondria which the diagram doesn't show as well. Thus a prokaryotic cell is shown in the image.
Clothes are left out to dry what is that an example of
Answers
Answer:
Evaporation
Explanation:
the process where a liquid (in this case water) is converted into a gas. Evaporation is a type of vaporization that occurs on the surface of liquid so it is a surface phenomenon
Non example of color change
Answers
Answer:
ion now i jus want points sorry
Explanation:
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
Which bone is located between the incus and the inner ear?
cochlea
stapes
incus
malleus
Answers
Answer: The answer is incus
Which of these is OK to wear in the lab?
Long, flowing, loose sleeves
Flip Flops
Bare midriff
Sandals
Long pants
Answers
Answer:
long pants
Explanation:
everything else is hazardous, and the long pants will protect your legs.
1. Mass of the empty Dish 167.0 g
2. Mass of the dish plus kernel before heating 169.0 g
3. Mass of the kernels before heating 2.0 g
4. Mass of the dish plus popped corn 168.8 g
5. Mass of the popped corn 1.8 g
6. Mass of the water driven 0.2 g
7. Mass percent of water in the popcorn 10%
Given that a sample of unpopped popcorn weighed 58.2 grams and after popping the popped kernels weighed 51.1 grams, calculate the percent water in the unpopped popcorn.
Answers
The mass of water driven off during popping can be calculated by subtracting the mass of the popped corn and the dish from the mass of the dish and kernel before heating.
What is heating ?Heating is the process of increasing the temperature of a substance or object, typically using an external energy source such as heat, radiation, or electrical current. The heat energy is transferred to the object or substance, causing its particles to vibrate and move faster, which results in an increase in temperature. Heating is commonly used in a wide range of applications, including cooking, chemical reactions, industrial processes, and space heating.
What is cooking?Cooking is the process of preparing food by applying heat, typically using methods such as baking, roasting, grilling, frying, boiling, simmering, steaming, or microwaving. The aim of cooking is to make food more palatable and easier to digest, as well as to kill harmful bacteria and other microorganisms that may be present in raw food. Cooking can also enhance the nutritional value of some foods by making certain nutrients more bioavailable.
To know more about heating visit :
https://brainly.com/question/1429452
#SPJ1
Across a period in the periodic table, atomic radii generally
Answers
\({ \blue{ \sf{decreases}}}\)
Explanation:
As we go across a period in the periodic table, atomic radii generally decreases because due to the increase in effective nuclear charge which tends to move towards itself i.e. the outermost electrons are pulled towards the nucleus and this leads to the atomic radii small.
Which of the following is a group of tissues that work together to carry out a
function?
A. Body system
B. Organs
C. Tissues
D. Organism
Answers
About how many miles across are high pressure areas
Answers
Answer: 30o N
30o N & S latitudes are high-pressure belts. Many of the world's deserts are situated at this latitude.
Explanation: Asia and North America witness the highest surface air pressure during winters. High-pressure areas in the clockwise north of the equator and counterclockwise in the south of the equator.
I will give Brainliest!!! Why did some scientists initially disagree that overall ice was decreasing?
Answers
Answer:
Human actions cannot change Earth's atmosphere . decrease in Earth's temperature . Although researchers at the polar station have now concluded that global average temperature has increased and that this is causing ice to melt, some scientists initially disagreed that the ice was melting .
Okay! Variability and Regional Differences:
Ice cover, whether it's sea ice, glaciers, or ice sheets, is subject to natural variability. Some scientists argued that the observed decreases in certain regions might be part of a natural cycle rather than a long-term trend. They pointed to historical records and paleoclimatic data, which suggested that ice cover has fluctuated in the past. This led to debates about whether the observed decreases were within the bounds of natural variability or indicative of a broader decline.
Data Limitations and Measurement Techniques:
Obtaining accurate and comprehensive data on ice cover is challenging. Satellite observations, which are crucial for monitoring large-scale ice changes, have limitations in terms of spatial and temporal coverage. Early satellite records had relatively short time spans, making it difficult to discern long-term trends. Additionally, different measurement techniques and instruments might introduce discrepancies in the data, which could lead to varying interpretations.
Discrepancies in Interpretation:
Scientists may differ in their interpretation of the available data due to varying expertise, perspectives, and biases. Some researchers may emphasize specific aspects of the data that align with their hypotheses or theories. This can contribute to differing conclusions about whether overall ice is decreasing or not.
Focus on Localized Increases:
While overall ice cover might be decreasing globally, localized increases in ice could complicate the assessment. For example, some regions might experience temporary gains in ice due to factors such as increased precipitation or changes in atmospheric circulation patterns. These localized increases can lead to debates about the net change in ice cover and its significance.
Publication Bias and Scientific Communication:
The process of scientific publishing can introduce biases. Scientists often publish research findings that deviate from the norm or present novel interpretations, leading to a disproportionate representation of dissenting views. Media coverage might amplify these discrepancies, creating the impression of a more significant scientific disagreement than actually exists.
It is important to note that the scientific consensus has evolved over time, and subsequent research and advancements in observational capabilities have provided clearer evidence of overall ice decline. Numerous scientific studies have since demonstrated a consistent decrease in ice cover across various regions, including Arctic sea ice, glaciers, and ice sheets like Greenland and Antarctica.
Scientists employ a rigorous process of peer review and critical analysis to evaluate research findings, resolve discrepancies, and refine our understanding of complex phenomena like ice decline. The gradual convergence of evidence from multiple sources, improved data quality, and the consensus-building process have led to a clearer understanding that overall ice is indeed decreasing.
It's worth emphasizing that the scientific process is inherently self-correcting, and ongoing research continues to refine our understanding of ice changes and their implications for our planet's climate system.
1.40 g H2 is allowed to react with 9.66 g N2, producing 2.24 g NH3
.
What is the theoretical yield in grams for this reaction under the given conditions?
Answers
The theoretical yield of NH₃ produced under the given conditions is 11.75 g.
The balanced equation for the reaction between hydrogen (H₂) and nitrogen (N₂) to form ammonia (NH₃) is;
N₂ + 3H₂ → 2NH₃
To determine the theoretical yield of NH₃ produced from the given amounts of H₂ and N₂, we need to calculate the limiting reactant and then use stoichiometry to find the maximum amount of NH₃ which can be produced.
The molar masses of H₂, N₂, as well as NH₃ are;
H₂; 2.02 g/mol
N₂; 28.02 g/mol
NH₃; 17.03 g/mol
The number of the moles of each reactant will be calculated as;
moles of H₂ = mass of H₂ / molar mass of H₂ = 1.40 g / 2.02 g/mol = 0.693 mol
moles of N₂ = mass of N₂ / molar mass of N₂ = 9.66 g / 28.02 g/mol = 0.345 mol
To determine the limiting reactant, we need to compare the mole ratios of H₂ and N₂ in the balanced equation with the actual mole ratios of the reactants. The balanced equation shows that 1 mole of N₂ will reacts with 3 moles of H₂ to produce a 2 moles of NH₃. The actual mole ratio of N₂ to H₂ in the reaction mixture is;
moles of N₂ / moles of H₂ = 0.345 mol / 0.693 mol
= 0.498
This ratio is less than the required ratio of 1/3, which means that N₂ is the limiting reactant. This means that all the N₂ will be consumed in the reaction and the amount of NH₃ produced will depend on the amount of N₂ present.
Using the mole ratio from the balanced equation, we can calculate the theoretical yield of NH₃ that can be produced from the 0.345 mol of N₂;
moles of NH₃ = (0.345 mol N₂) × (2 mol NH3 / 1 mol N₂)
= 0.690 mol NH₃
The mass of this amount of NH₃ can be calculated as;
mass of NH₃ = moles of NH₃ × molar mass of NH₃ = 0.690 mol × 17.03 g/mol = 11.75 g
Therefore, the theoretical yield is 11.75 g.
To know more about theoretical yield here
https://brainly.com/question/14966377
#SPJ1
Given the problem below, what would the first step be in solving it?
How many grams of NaCl will be produced from 18.0 g of Na and 23.0 g Cl2?
2Na + Cl2 --> 2NaCI
Question 3 options:
Convert to moles
Use the mole ratio
Start with the given information 18 g Na and 23 g Cl2
Convert to grams
Answers
Answer:
First step would be convert to moles
Final Answer: 37.8 g of NaCl
Explanation:
The reaction is:
2Na + Cl₂ → 2NaCI
We convert the mass of each reactant to moles:
18 g . 1mol /23g = 0.783 moles of Na
23g . 1mol / 70.9g = 0.324 moles of chlorine
We use the mole ratio to determine the limiting reactant:
Ratio is 2:1. 2 moles of Na react to 1 mol of chlorine
Then, 0.783 moles of Na, may react to (0.783 . 1)/2 = 0.391 moles.
Excellent!. We need 0.391 moles of Cl₂ and we only have 0.324 moles available. That's why the Cl₂ is our limiting reactant.
We use the mole ratio again, with the product side. (1:2)
1 mol of Cl₂ can produce 2 moles of NaCl
Then, our 0.324 moles of gas, may produce (0.324 . 2)/1 = 0.648 moles
Finally, we convert the moles to grams:
0.648 mol . 58.45g/mol =
Florian is testing some household supplies with red and blue litmus paper. The results are in the table below. A 3-column table with 3 rows. The first column labeled sample has entries glass cleaner, aspirin dissolved in water, sugar water. The second column labeled red litmus has entries red, blue, blue. The third column labeled blue litmus has entries blue, red, blue. What can he conclude about the pH of each sample? Glass cleaner is . Aspirin is . Sugar water is .
Answers
Answer:
Glass cleaner is: acidic
Aspirin is: basic
Sugar water is: neutral
Answer:
basic
acidic
nuetral
Explanation:
in that order
PLEASE HELP QUICK!
Your organ systems do not function
independently. Consider the following
scenarios, and explain how different
organ systems are working together.
a) You step on hot pavement in the
summer and quickly pull your foot
back.
(b) after running up the stairs you
breathe a little harder.
(c) A friend has a cold, and three days
later you have a cold too.
Answers
Answer:A
Explanation:
Your muscular system is at work
What problem is associated with high stability of polymers?
Answers
Explanation:
they do not break down quickly or easily and create a lot of pollution (like microplastics)