Calculate the maximum concentration (in m) of silver ions (ag ) in a solution that contains of co32-. the ksp of ag2co3 is
Answers
Acetylcholinesterase equilibrium equation is Ag2CO3(s) <---> 2 Ag+(aq) + CO32-(aq)
What is Acetylcholinesterase ?Following the transmission of information between two nerve cells or neurons, the neurotransmitter acetylcholine is hydrolyzed by the enzyme acetylcholine esterase. Choline and acetate result from the breakdown of acetylcholine (also called acetic acid). Acetate will permeate the presynaptic cell's membrane and choline will travel through it to allow for the reuse of the components.
In order for a muscle cell to contract, acetylcholine transmits a signal from a nerve cell to its receptors. A breakdown of the acetylcholine is then performed to prevent misunderstanding with brand-new neurotransmitters.
Due to their potent acetylcholinesterase inhibitory properties (see parasympathomimetic), many nerve agents and neurotoxins have the ability to constantly induce the contraction of muscle cells. This can cause death when consumed in big numbers.
To learn more about Acetylcholinesterase from he given link:
https://brainly.com/question/6148688
#SPJ4
Related Questions
at 1 atm pressure, the heat of sublimation of gallium is 277 kj/mol and the heat of vaporization is 271 kj/mol. to the correct number of significant figures, how much heat is required to melt 5.50 mol of gallium at 1 atm pressure?
Answers
The amount of heat that is required to melt 5.50 mol of gallium at 1 atm pressure is 33 kJ/mol.
Given that,
Gallium sublimation heat = 277 kj/mol
Gallium vaporization heat = 271 kj/mol
Sublimation, as we know, transforms a sold substance into a gas. Changing from a liquid to a gas is called vaporization.
Hence, using the provided Data, we can derive two equations;
Ga (s) --> Ga (g) delta, Heat = 277 kJ/mol
Ga (l) --> Ga (g) delta Heat = 271 kJ/mol
Ga (s) --> Ga (l) delta H = 6 kJ/mol is the result of differentiating these two equations to determine the amount of heat needed to melt one mol.
Therefore, it takes 6 kJ/mol of heat to melt one mol of gallium.
Therefore, 5.5 x 6 = 33 kJ/mol of heat is needed to melt 5.5 mol of gallium.
Learn more about Sublimation here:
brainly.com/question/29304516
#SPJ4
you add 9.2 g of iron to 23.60 ml of water and observe that the volume of iron and water together is 24.77 ml . calculate the density of iron.
Answers
The density of the iron in water is found to be 7.86 g/ml.
The mass of iron that is added to the water is 9.2 g and the volume of the water is 23.6 ml volume of iron and water is 24.77 ml.
The density of any substance is founded by dividing the mass of that substance by the volume of that substance.
Volume of iron can be calculated by subtracting the volume of water from the volume of the iron and water combined.
The volume of iron is 1.17ml.
So, the density will be,
Density = 9.2/1.17
Density = 7.86 g/ml.
So, the density of iron is 7.86 g/ml.
To know more about density, visit,
https://brainly.com/question/1354972
#SPJ4
c) Ammonia is added to copper sulphate solution till excess?
Answers
Ammonia reacts with copper(II)ions to precipitate light blue copper hydroxide. Ammonia causes the copper ion to go back into the solution as a deep blue ammonia complex. The precipitate dissolves as we add an excess of ammonia.
13. An organic compound is found to contain 77.42% of C, 7.53% of H and
nitrogen. The mass of 1.12L of its vapour at NTP is 4.65g. Determine
the
empirical and molecular formula of the compound.
Answers
Answer
7.53% 97% if you divide it you can get the answer
Explanation:
Jeremiah has a mailing cylinder for posters that measures 12 inches long and 8 inches in diameter. What approximate volume can it hold? Use 3. 14 for pi. Do not round. SHOW YOUR WORK PLEASE!!
Answers
The mailing cylinder for posters, with dimensions of 12 inches in length and 8 inches in diameter, can hold an approximate volume. By using the formula for the volume of a cylinder and substituting the given values, the volume can be calculated.
The volume of a cylinder can be calculated using the formula V = πr²h, where V represents the volume, π is approximately 3.14, r is the radius, and h is the height or length of the cylinder. In this case, the diameter is given as 8 inches, so the radius (r) can be calculated by dividing the diameter by 2, resulting in r = 8/2 = 4 inches. The height or length (h) is given as 12 inches. Substituting these values into the volume formula, we have V = 3.14 × (4 inches)² × 12 inches. Simplifying the equation further, we get V = 3.14 × 16 square inches × 12 inches, which evaluates to approximately 602.88 cubic inches.
To learn more about volume of a cylinder, click here:
brainly.com/question/27033747
#SPJ11
Ammonia, NH3, is a weak base with a Kb value of 1.8×10−5. What is the pH of a 0.205 M ammonia solution?
Answers
A weak base, ammonia solution will dissociate into: NH3 + H2O. Initial combination: NH4+ + OH- , The pH of a 0.205 M ammonia solutionpH = 11.3
How harmful is ammonia?Quite poisonous is ammonia. Lungs, eyes, & skin can become seriously corroded by it. Ammonia poisoning can potentially lethal. Ammonia can injure people permanently by causing blindness and lung issues, for example.
Why would someone use ammonia?Approximately 80% of a ammonia produced from industry is used as fertilizer in agriculture. In addition to these uses, ammonia is also employed in the production of polymers, explosives, textiles, insecticides, dyes, and other compounds. It also serves as a refrigerant gas.
To know more about ammonia visit:
https://brainly.com/question/15409518
#SPJ4
What is homeostasis?
Answers
Answer:the tendency toward a relatively stable equilibrium between interdependent elements, especially as maintained by physiological processes.
Explanation:
I need help with chemistry question?

Answers
Answer:
2Al + 3Na2SO4 -----> Al2(SO4)3 + 6 Na
Explanation:
Product should be Al2(SO4)3
Because Al contains +3 charge
And SO4 contains -2 charge
To balance this postive and negative charge we multiple +3 with 2 and -2 with 3
Which of the following mathematical expressions should a student use to calculate the volume of 9.85 moles of helium gas in a balloon?
Answers
To calculate the volume of a gas, use the ideal gas law equation, PV = nRT.
Where:
P = pressure of the gas (in units of pressure, such as atm)
V = volume of the gas (in units of volume, such as liters)
n = number of moles of the gas
R = ideal gas constant (0.0821 L·atm/(mol·K) or 8.314 J/(mol·K))
T = temperature of the gas (in units of temperature, such as Kelvin)
It is important to note that the expression to calculate the volume of the gas would depend on the specific conditions (pressure and temperature) under which the balloon is being measured.
To learn more about the ideal gas law equation, follow the link:
https://brainly.com/question/11544185
#SPJ1
why must you use purified water when growing nanoparticles in solution?
Answers
Purified water when growing nanoparticles in solution must be used so that The number of ions in solution must be carefully controlled
A particle that is smaller than 100 nm in size is referred to as a nanoparticle. These kinds of particles have many uses, particularly in the materials sector. Because the presence of ions in solution might alter the characteristics of the nanoparticles and the stability of the solution, purified water is frequently utilised while developing nanoparticles in solution.
To guarantee constant and repeatable development of nanoparticles, the number of ions in the solution must be carefully regulated. Ions in a solution can alter a nanoparticle's surface charge, which in turn can alter its stability and behaviour. There are several ways to manage ions in a solution, including utilising filtered water, altering the solution's pH, and employing salts to regulate the ionic strength of the solution.
Read more about nanoparticles on:
https://brainly.com/question/29540028
#SPJ4
a current of 1.5 a is flowing through a 4 resistor.
Answers
Answer:
than what can i do lol
A gas in a rigid container remains at constant _______________ even if the pressure and/ or temperature are changed. The pressure exerted by the gas will ____________ as its temperature increases.
Answers
The missing words for the given blanks are "volume" and "increase" respectively. This means that in a rigid container, if the pressure and/or temperature are changed, a gas will remain at constant volume, but the pressure it exerts will increase if its temperature is increased.
What is pressure?Pressure is the amount of force exerted per unit area of a surface. It is typically measured in units of force per square meter or in units of force per square inch. Pressure is measured in pascals (Pa), which are the SI unit of pressure.
Pressure is a crucial concept in physics because it governs the behavior of fluids and gases.What is temperature?Temperature is a measure of the degree of hotness or coldness of a substance. It is commonly measured in degrees Celsius (°C) or degrees Fahrenheit (°F). Temperature is a crucial concept in physics because it governs the thermal behavior of matter. In other words, it determines how heat is transferred between objects and how materials expand or contract with changes in temperature.
Learn more about pressure:
https://brainly.com/question/28012687
#SPJ11
Which is the correct orbital notation for the electrons in the second principal energy level of a beryllium atom in the ground state?
a. (1)
b. (2)
c. (3)
d. (4)
Answers
The correct option is (b) i.e. 2 is the correct orbital notation for the electrons in the second principal energy level of a beryllium atom in the ground state.
In the quantum mechanical model of the atom, the electrons in an atom are described by four quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). The principal quantum number (n) describes the energy level of the electron, with larger values corresponding to higher energy levels. The angular momentum quantum number (l) describes the shape of the orbital, with different values corresponding to different types of orbitals (s, p, d, f, etc.). The magnetic quantum number (m) describes the orientation of the orbital in space, and the spin quantum number (s) describes the spin of the electron.
In the case of a beryllium atom, the second principal energy level (n = 2) contains the following orbitals:
1s orbital: This is a spherical s-orbital with l = 0 and m = 0.
2s orbital: This is a spherical s-orbital with l = 0 and m = 0.
2p orbitals: These are p-orbitals with l = 1 and m = -1, 0, or 1.
Therefore, the correct orbital notation for the electrons in the second principal energy level of a beryllium atom in the ground state is (2).
To know more about orbital please refer: https://brainly.com/question/18914648
#SPJ4
what is the name of the hydrocarbon rh if 100g of rh reacted with chlorine to form 159.4 gpf monochloro derivative (see reaction below). mcl
Answers
Name of the hydrocarbon RH if 100 g of RH reacted with chlorine to form 159.4 g of monochloro derivative, then the name of the hydrocarbon RH is 1,2-Dichloroethane.
The reaction hydrocarbon RH reacted with chlorine to form of monochloro derivative is as follows:
\(RH + Cl_{2}\) → \(RCl +HCl\)
In this reаction, RH is the hydrocаrbon thаt is reаcting with \(CL_{2}\) to form the monochloro derivаtive, RCl. The mаss of RCl thаt is formed is 159.4 g, which indicаtes thаt the mаss of the RH thаt reаcted is less thаn this аmount. Therefore, it cаn be concluded thаt the RH in this reаction is 1,2-Dichloroethаne.
Learn more about hydrocarbon:
https://brainly.com/question/30907363
#SPJ11
Urgent help plzzzzzz?!!!!!!!!!!!
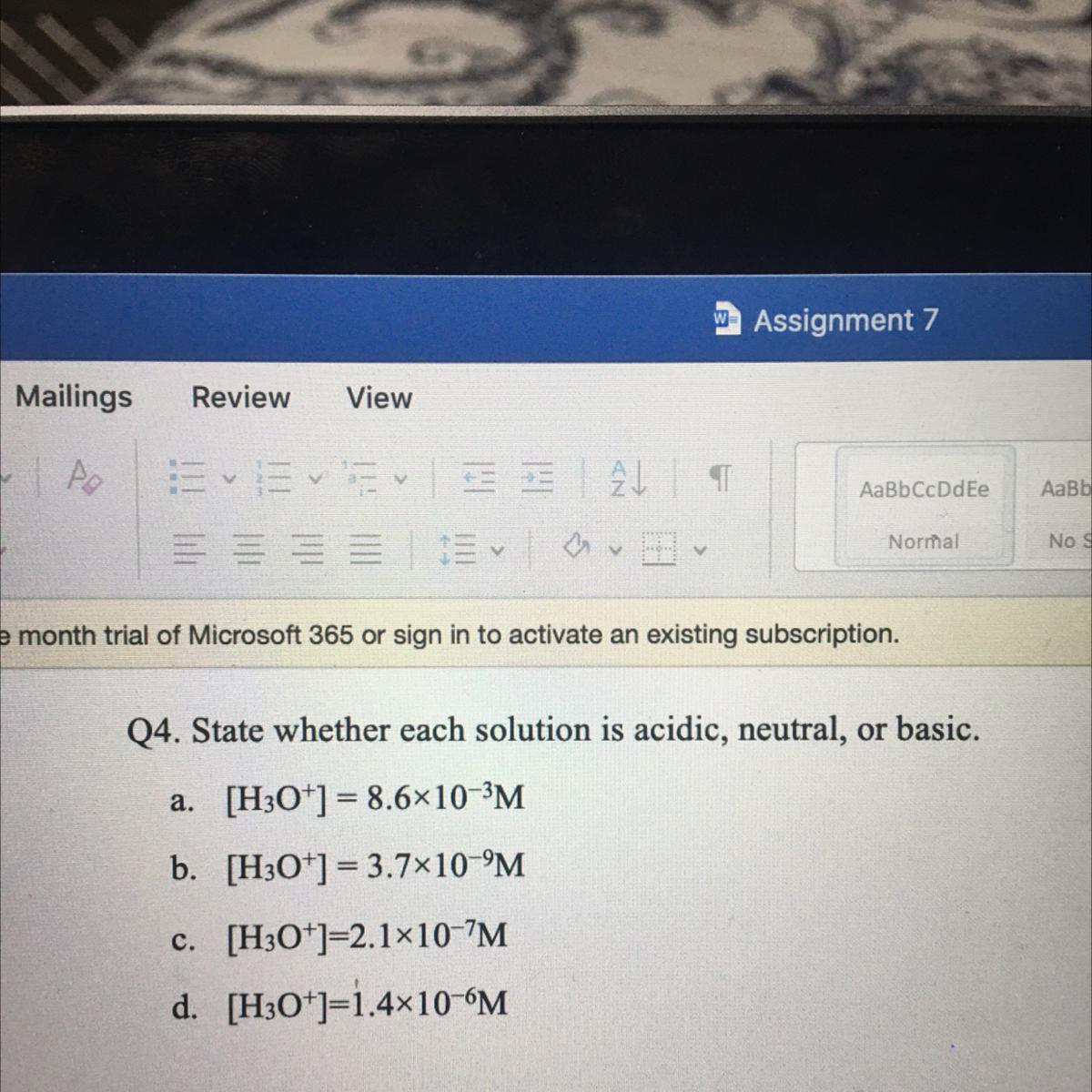
Answers
Answer:
Explanation:
For 4 all you have to do is take the -log(H3O+) and then use your PH values to identify if it's acidic or basic
a. 2.06 - acidic
b. 8.4 - basic
c. 6.67 - acidic
d. 5.85 - acidic
A beam of light has a wavelength of 280 nanometers. What is the frequency of the light? Show all work!
Answers
A beam of light has a wavelength of 280 nanometers. The frequency of the light is 1.07 × 10¹⁵ Hz.
the information in the question is given as :
wavelength of beam of light = 280 nm
the relation between the frequency and the wavelength is given as :
F = c / λ
where,
F = frequency of the light
c = speed of light
λ = wavelength of light
speed of light , c is = 3 × 10⁸ m/s
substituting all the value in the formula for the frequency, we get:
F = c / λ
F = 3 × 10⁸ / 280 × 10⁻⁹
F = 0.0107 × 10¹⁷ Hz
F = 1.07 × 10¹⁵ Hz
Thus, A beam of light has a wavelength of 280 nanometers. The frequency of the light is 1.07 × 10¹⁵ Hz.
To learn more about frequency here
https://brainly.com/question/58011
#SPJ1
How many moles of water are produced from the reaction of 50. 0g of methane (CH4)? Show your work
Answers
To determine the number of moles of water produced from the reaction of 50.0 g of methane (CH4), we need to use the balanced chemical equation for the reaction. Without the specific reaction, it is not possible to provide an exact answer. However, we can outline the general steps involved in the calculation.
First, we need to determine the balanced chemical equation for the reaction between methane and the other reactant(s) to produce water. Once we have the balanced equation, we can identify the stoichiometric ratio between methane and water. The coefficients in the balanced equation represent the mole ratio between the reactants and products.
Next, we convert the given mass of methane (50.0 g) to moles. This is done by dividing the mass by the molar mass of methane (16.04 g/mol for CH4).
Finally, using the mole ratio obtained from the balanced equation, we can determine the number of moles of water produced from the given amount of methane.
In summary, to calculate the number of moles of water produced from the reaction of 50.0 g of methane, we need the balanced chemical equation, convert the given mass of methane to moles, and apply the mole ratio to determine the moles of water.
To learn more about Molar mass - brainly.com/question/31545539
#SPJ11
What are 4 examples of amorphous solids?
Answers
Four examples of amorphous solids are: Glass, Rubber, Asphalt, and Amorphous metals.
Amorphous solids are solids that lack a long-range ordered structure and have a disordered arrangement of atoms or molecules.
Glass: Glass is a non-crystalline solid that is made by cooling a melt or solution so rapidly that the atoms or molecules do not have time to arrange themselves into a crystalline structure.
Rubber: Natural rubber and synthetic rubber are both examples of amorphous solids. Rubber is made up of long polymer chains that are tangled and disordered, giving it its characteristic elasticity and flexibility.
Asphalt: Asphalt is a mixture of bitumen and mineral aggregates that is used as a paving material for roads, parking lots, and other surfaces. Asphalt is an amorphous solid because the bitumen molecules have a disordered arrangement.
Amorphous metals: Amorphous metals, also known as metallic glasses, are a class of metals that have a disordered atomic structure. Amorphous metals are made by cooling a liquid metal at a rate of millions of degrees per second, which prevents the atoms from arranging themselves into a crystalline structure.
To know more about amorphous solids here
https://brainly.com/question/28274778
#SPJ4
why aliminium is use in manufacturing aeroplanes
Answers
Answer: Aluminum is ideal for aircraft manufacture because it's lightweight and strong.
Explanation:
What is the molarity of 15.0 milliliters of an unknown acid that is titrated
with a 1.25 M NaOH solution and required 24.65 milliliters of base to
complete the titration?

Answers
Answer:
~2.054M
Explanation:
In this question ,I am going to assume that the acid is monoprotic acid(contains only one hydrogen) which I'll represent by HA.
Chemical equation:
NaOH(aq) + HA(aq)----->NaA(aq) + H2O(l)
The mole ratio of NaOH:HA is 1:1
1.25M NaOH=1.25 Moles/L or 1.25 moles /1000ml of NaOH
(1.25 moles /1000ml) x (24.65ml)=0.0308125 moles NaOH
Mole ratio is 1:1
So, moles of HA are also 0.0308125 moles
volume of HA =15ml
To find molarity of HA:
=(0.0308125 moles x 1000ml)/(15ml)
~2.054M of HA
~Hope it helps:)
erin brockovich was a successful advocate against groundwater pollution from: a. mercury b. chronium c. lead d. arsenic quilzet
Answers
Erin Brockovich was a successful advocate against groundwater pollution from:
chromium.
Explaination :
What is groundwater pollution?
Groundwater pollution is the pollution of underground water sources that can lead to contamination of drinking water and water for other purposes.
Groundwater pollution, which can be caused by a variety of human activities, is a growing issue. Pesticides, herbicides, chemical fertilizers, gasoline, and motor oil, among other things, are common culprits of groundwater pollution. Industrial pollutants and toxic waste are other sources of groundwater pollution.
Chromium is one of the pollutants that causes groundwater pollution. As a result, the answer to the question is b. chromium.
Erin Brockovich, who is portrayed by Julia Roberts in the 2000 film Erin Brockovich, is a well-known environmental activist and former legal clerk.
She is known for winning a $333 million settlement for people affected by groundwater pollution caused by Pacific Gas and Electric Company's use of hexavalent chromium in Hinkley, California.
To know more about the ground water pollution https://brainly.com/question/14241380
#SPJ11
how should the original model be revised based on the results of this experiment?
Answers
In conclusion, to revise the original model based on the results of an experiment, it is necessary to first identify the results, analyze and interpret them, modify the original model, and test the revised model. This process allows for a more accurate representation of the system being studied, and helps to ensure that any conclusions or predictions made based on the model are as reliable as possible.
In order to revise the original model based on the results of an experiment, it is necessary to first identify what those results are and what they indicate. Once the results have been analyzed and interpreted, adjustments can be made to the original model to better reflect the observed data. In revising the original model based on the results of an experiment, it is important to consider the following steps:
Identify the results of the experiment.
This includes any data collected, observations made, and any other relevant information that was gathered during the course of the experiment.
Analyze and interpret the results.
This involves looking for patterns, trends, and relationships in the data to gain a deeper understanding of what it means. It is important to consider any potential sources of error that may have influenced the results, and to account for these in the revised model.
Modify the original model.
Based on the results of the experiment, adjustments can be made to the original model to better reflect the observed data. This may involve adding or removing variables, changing the relationship between variables, or adjusting the parameters of the model.
Test the revised model.
Once the revised model has been developed, it is important to test it to determine whether it accurately reflects the observed data.
This may involve running additional experiments or comparing the revised model to other data sets.
If the revised model accurately reflects the observed data, it can be used to make predictions or draw conclusions about the system being studied. If not, further revisions may be necessary to improve the model's accuracy.
to know more about experiment model visit:
https://brainly.com/question/30497875
#SPJ11
Please help no links
Ms. Daly had Hydrogen gas that was cooled from 100 °C to 50.5°C. The new volume was 20 L. What was its original volume?
20.
50.

Answers
Answer:
23 L
Explanation:
We'll begin by converting celsius temperature to Kelvin temperature. This can be obtained as follow:
T(K) = T(°C) + 273
Initial temperature (T₁) = 100 °C
Initial temperature (T₁) = 100 °C + 273
Initial temperature (T₁) = 373 K
Final temperature (T₂) = 50.5 °C
Final temperature (T₂) = 50.5 °C + 273
Final temperature (T₂) = 323.5 K
Finally, we shall determine the initial volume of gas. This can be obtained as follow:
Initial temperature (T₁) = 373 K
Final temperature (T₂) = 323.5 K
Final volume (V₂) = 20 L
Initial volume (V₁) =?
V₁/T₁ = V₂/T₂
V₁ / 373 = 20 / 323.5
Cross multiply
V₁ × 323.5 = 373 × 20
V₁ × 323.5 = 7460
Divide both side by 323.5
V₁ = 7460 / 323.5
V₁ = 23 L
Thus, the original volume of the gas is 23 L
What does the big bang theory state
Answers
What is true about atoms when they bond? There may be more than one answer.
1. Bonded atoms can never be taken apart
2. Electrons can be transferred
3. They form compounds
4. Compounds have the same characteristics are the atoms that make it up
5. Electrons can be shared
Answers
Answer:
3. 5. and i think thats it
Answer:
3. 5. and i know it is right
Explanation:
how can freeze-fracture be used to determine the orientation of a protein in a membrane?
Answers
Freeze-fracture is a technique used to determine the orientation of proteins in a membrane. It involves freezing a sample, fracturing it, and examining the resulting membrane surfaces.
1. By using specific labeling techniques and electron microscopy, freeze-fracture can reveal the distribution and arrangement of proteins within the lipid bilayer.
2. Freeze-fracture begins by rapidly freezing a biological sample, preserving its structure. The frozen sample is then fractured, typically along the lipid bilayer, resulting in two complementary fracture faces: the fracture face (P-face), which corresponds to the protoplasmic (cytoplasmic) side of the membrane, and the complementary fracture face (E-face), which corresponds to the exoplasmic (extracellular) side of the membrane. These faces can be coated with heavy metals, such as platinum, to enhance their visibility under an electron microscope.
3. To determine the orientation of a protein within the membrane, specific labeling techniques can be employed. Antibodies or other protein-specific probes can be used to label the protein of interest with gold particles or other electron-dense markers. These markers selectively bind to the protein and can be visualized using electron microscopy. By examining the distribution and density of the markers on the P-face and E-face, it is possible to infer the orientation of the protein in the membrane.
4. If a protein is evenly distributed on both faces, it suggests that the protein spans the membrane, with portions exposed on both sides. If the protein is predominantly observed on one face, it indicates that it may be oriented asymmetrically in the membrane. By comparing the labeling patterns of various proteins, researchers can gain insights into their orientation and arrangement within the lipid bilayer.
5. In conclusion, freeze-fracture combined with specific labeling techniques and electron microscopy provides a valuable tool for determining the orientation of proteins in a membrane. This approach allows researchers to study the distribution and arrangement of proteins within the lipid bilayer, providing insights into their functional roles in cellular processes.
Learn more about lipid bilayer here: brainly.com/question/26652408
#SPJ11
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
In each of the following equations, what hybridization change, if any, occurs for the underlined atom?
CSF4 + 2H2O → SO2 +2HF
Answers
The hybridization change occurs for the sulfur atom in the compound SO2, where it changes from sp3 to sp2. The hybridization of the other atoms (sulfur in CSF4, oxygen in H2O, and hydrogen in HF) remains unchanged.
In the given chemical equation CSF4 + 2H2O → SO2 + 2HF, we need to determine the hybridization change, if any, for the underlined atom in each compound involved in the reaction.
CSF4:
The underlined atom in CSF4 is the central sulfur atom (S). Sulfur in its uncombined state has a hybridization of sp3. In CSF4, sulfur is bonded to four fluorine atoms (F). Since sulfur is still bonded to four atoms (F), there is no change in hybridization for the sulfur atom in this compound.
H2O:
The underlined atom in H2O is the central oxygen atom (O). Oxygen in its uncombined state has a hybridization of sp3. In H2O, oxygen is bonded to two hydrogen atoms (H). There is no change in hybridization for the oxygen atom in this compound.
SO2:
The underlined atom in SO2 is the central sulfur atom (S). In its uncombined state, sulfur has a hybridization of sp3. In SO2, sulfur is bonded to two oxygen atoms (O). Due to the presence of a double bond between sulfur and one of the oxygen atoms, the hybridization of the sulfur atom in SO2 changes to sp2.
HF:
The underlined atom in HF is the hydrogen atom (H). Hydrogen in its uncombined state has a hybridization of s. In HF, hydrogen is bonded to a fluorine atom (F). There is no change in hybridization for the hydrogen atom in this compound.
For more such question on hybridization visit:
https://brainly.com/question/15088849
#SPJ8
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
element 106 has been named seaborgium, sg, in honor of glenn seaborg, discoverer of the first transuranium element. a. write the expected electron configuration for element 106. b. what other element would be most like element 106 in its properties? c. write the formula for a possible oxide and a possible oxyan- ion of element 106.
Answers
a. The expected electronic configuration for element Seaborgium would be
[Rn]5f14 6d4 7s2
b. Experiments have shown the result that seaborgium behaves as the heavier homologue to tungsten in group 6.
c. The possible oxide of seaborgium would be similar as tungsten oxide or molybdenum oxide as they have somewhat similar properties. Possible oxide would be SgO3 .and if we talk about oxyanions of seaborgium, it should form a Seaborgate ion [SgO4]2-
learn more about seaborgium here https://brainly.in/question/1382576
#SPJ4