calculate the heart rate of an individual with a cardiac output of 7.0 l and a stroke volume of 70 ml/min
Answers
To calculate the heart rate of an individual with a cardiac output of 7.0 L and a stroke volume of 70 mL/min, we need to use the formula: Cardiac Output (CO) = Stroke Volume (SV) x Heart Rate (HR).
First, we need to convert the stroke volume from ml/min to liters: 70 mL/min = 0.07 L/min
Now we can plug in the values: 7.0 L/min = 0.07 L/min x HR, To solve for HR, we need to divide both sides by 0.07L/min: HR = 7.0 L/min ÷ 0.07 L/minmHR = 100 beats per minute, Therefore, the heart rate of this individual would be 100 beats per minute, given a cardiac output of 7.0 L and a stroke volume of 70 ml/min.
Now, you can plug in the values into the formula: Heart Rate = 7.0 L/min / 0.07 L/beat
Heart Rate ≈ 100 beats/min, So, the individual's heart rate is approximately 100 beats per minute.
To know more about formula click here
brainly.com/question/29886204
#SPJ11
To calculate the heart rate of an individual with a cardiac output of 7.0 L and a stroke volume of 70 ml/min, you'll need to use the following formula:
Heart Rate = Cardiac Output / Stroke Volume
Step 1: Convert the cardiac output and stroke volume to the same units. In this case, let's convert the cardiac output to milliliters (mL). Since 1 L equals 1000 mL, the cardiac output is 7.0 L * 1000 mL/L = 7000 mL.
Step 2: Plug the values into the formula:
Heart Rate = 7000 mL (Cardiac Output) / 70 mL/min (Stroke Volume)
Step 3: Calculate the heart rate:
Heart Rate = 7000 mL / 70 mL/min = 100 beats/min
So, the heart rate of the individual is 100 beats per minute.
To know more about Heart rate Calculation:
https://brainly.com/question/871799
#SPJ11
Related Questions
Cobalt-60 is produced by a three reaction process involving neutron capture, beta-emission, and neutron capture. The initial reactant in the production of cobalt-60 is ________.
Answers
The initial reactant in the production of cobalt-60 is cobalt-59.
This isotope of cobalt is bombarded with neutrons, which causes it to undergo neutron capture, resulting in cobalt-60. The cobalt-60 then undergoes beta-emission, which converts a neutron into a proton and releases a beta particle.
Finally, another neutron is captured by the cobalt-60 to produce the stable isotope nickel-60. This three-reaction process results in the production of cobalt-60, which is a radioactive isotope used in medical and industrial applications.
To know more about cobalt-60 click on below link :
https://brainly.com/question/12517036#
#SPJ11
Acetic acid (CH 3 COOH) reacts with water to form the acetate ion and the hydronium ion: CH 3 COOH(aq)+H 2 O(l) leftrightarrow CH 3 COO^ - (aq)+H 3 O^ + (aq) At equilibriumthe concentration of CH 3 COOH is 2. 0 * 10 ^ - 1 * M the concentration of CH 3 COO^ - 1. 9 * 10 ^ - 3 * M and the concentration of H 3 O^ + is 1. 9 * 10 ^ - 3 * M What is the value of K eq for this reaction? 1. 8 * 10 ^ - 5 5. 5 * 10 ^ 4 9. 5 * 10 ^ - 3 1. 1 * 10 ^ 2
Answers
The value of K_eq for this reaction is approximately 1.805 × 10^-5.
To find the value of the equilibrium constant (K_eq) for the given reaction, we can use the equilibrium concentrations of the species involved. The equilibrium constant expression for the reaction is:
K_eq = [CH3COO-][H3O+] / [CH3COOH]
Given the following concentrations at equilibrium:
[CH3COOH] = 2.0 × 10^-1 M
[CH3COO-] = 1.9 × 10^-3 M
[H3O+] = 1.9 × 10^-3 M
Substituting these values into the equilibrium constant expression, we get:
K_eq = (1.9 × 10^-3)(1.9 × 10^-3) / (2.0 × 10^-1)
K_eq = 3.61 × 10^-6 / 2.0 × 10^-1
K_eq = 3.61 × 10^-6 × 5.0 × 10^0
K_eq = 1.805 × 10^-5
Therefore, the value of K_eq for this reaction is approximately 1.805 × 10^-5.
None of the provided answer choices match this value exactly.
For such more questions on K_eq
https://brainly.com/question/13997800
#SPJ8
if 6 moles of a a compound produce 84 J of energy, what is the h reaction in j/mol
Answers
The enthalpy of the reaction is 14 J/mol.
The enthalpy of a reaction (ΔH) is the amount of energy transferred between a system and its surroundings during a chemical reaction at constant pressure, measured in joules per mole (J/mol). This value is important because it can tell us whether a reaction is exothermic or endothermic, as well as give us information about the strength of chemical bonds within the reactants and products.To calculate the enthalpy of a reaction, we need to know the amount of energy released or absorbed (Q) and the number of moles of the compound involved in the reaction (n). We can use the equation:
ΔH = Q/n
Given that 6 moles of a compound produce 84 J of energy, we can calculate the enthalpy of the reaction as follows:
ΔH = Q/n
ΔH = 84 J / 6 mol
ΔH = 14 J/mol
This means that for every mole of the compound involved in the reaction, 14 J of energy is transferred between the system and the surroundings. Since the value is positive, we can conclude that the reaction is endothermic, meaning that it requires an input of energy to occur.It is worth noting that the enthalpy of a reaction can depend on a number of factors, such as temperature, pressure, and the specific conditions under which the reaction occurs. As such, it is important to take these factors into account when calculating or predicting enthalpy values.
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
In an experiment, the one variable that is changed is called the
Answers
help me please !! is it A,B,C, or D ???

Answers
A. Is indicated by a negative enthrall sign.
Reasoning: an endothermic reaction is ice melting and the energy being more than its surroundings. Not specified to ice but as an example, ice is endothermic. That puts d and b out of the running leaving you left with a and c.
When I searched up enthalpy, it said “When a substance changes at constant pressure, enthalpy tells how much heat and work was added or removed from the substance.” Which is similar to c, right? Yeah, meaning both a and c are similar in that aspect.
The reason I decided to go with a is because heat is NOT released into the surrounding, exothermic reactions release energy and heat into the surrounding.
why is it useful to group large number of things?
Answers
mole is a very important unit of measurement that chemists use. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs means you have twelve eggs. Chemists have to measure using moles for very small things like atoms, molecules, or other particles.
For 2 hours, Leah was driving east at one-half of her car's top speed. Her car can go at a maximum speed of 180 kilometers per hour. In this time, how far did Leah drive?
Answers
Answer:
180 kilometers in 2 hours.
Explanation:
To find the distance Leah drove, we need to first determine her average speed. Since Leah was driving east at one-half of her car's top speed, her average speed was 180/2 = 90 kilometers per hour.
Next, we can use this speed to determine the distance she traveled. Since Leah was driving for 2 hours at an average speed of 90 kilometers per hour, she traveled 2 * 90 = 180 kilometers.
Therefore, Leah drove 180 kilometers in 2 hours while driving east at one-half of her car's top speed.
The base protonation constant Kb of trimethylamine ((CH3)3N) is 6.31x io Calculate the pH of a 0.36 M solution of trimethylamine at 25 °C. Round your answer to 1 decimal place.
Answers
Answer: The protonation of trimethylamine can be represented by the following equilibrium reaction:
(CH3)3N + H2O ⇌ (CH3)3NH+ + OH-
The equilibrium constant for this reaction, which is the base ionization constant (Kb) of trimethylamine, is 6.31 x 10^-5 at 25°C.
The Kb expression for this reaction is:
Kb = [ (CH3)3NH+ ][OH-] / [(CH3)3N]
At equilibrium, we can assume that [OH-] = [ (CH3)3NH+ ] since one mole of hydroxide ion is produced for every mole of trimethylamine that is protonated. Therefore, we can simplify the Kb expression to:
Kb = [ (CH3)3NH+ ]^2 / [(CH3)3N]
We can rearrange this expression to solve for [ (CH3)3NH+ ]:
[ (CH3)3NH+ ] = sqrt(Kb * [(CH3)3N])
Plugging in the given values, we get:
[ (CH3)3NH+ ] = sqrt(6.31 x 10^-5 * 0.36 M) = 0.0104 M
The concentration of hydroxide ion in the solution is also equal to [ (CH3)3NH+ ] since the reaction produces one mole of hydroxide ion for every mole of trimethylamine that is protonated.
pOH = -log[OH-] = -log[ (CH3)3NH+ ] = -log(0.0104) = 1.98
Using the relation pH + pOH = 14, we get:
pH = 14 - pOH = 14 - 1.98 = 12.02
Therefore, the pH of the 0.36 M solution of trimethylamine is 12.0 (rounded to 1 decimal place).
Which set of elements contains a metalloid?
Oa. Ba, Ag, Sn, Xe
O b. Fr, F, O, Rn
O c. Li, Mg, Ca, Kr
O d. K, Mn, As, Ar
Answers
Answer:
D.) K, Mn, As, Ar
Explanation:
The metalloids are located in the p-block on the periodic table and have a ladder-like arrangement.
The most commonly recognized metalloids are:
boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te)
if 100.0 mL of liquid weighs 81.23g what is the density of the liquid
Answers
Answer:
812.3 kilogram/cubic meter
Explanation:
I used an online calculator.
Hope I helped!
given the following data C =66.7% H =11.1% Calculate the empirical formula of the compund
Answers
First, we calculate the moles of each element taking the percentages as a mass:
\(66,7g\text{ C}\cdot\frac{1\text{ mol C}}{12\text{ g C}}=5,56\text{ mol C}\)\(11,1\text{ g H}\cdot\frac{1\text{ mol H}}{1\text{ g H}}=11,1\text{ mol H}\)We divided the number of moles by the smaller number of moles. In this case, C is the smallest:
\(5,56\text{ mol C/5,56 =1}\)\(11,1\text{ mol H/5,56=1,99}\approx2\)These numbers give us the empirical formula wich is: CH2
Jayadev has apassion for photography. Maker the there films out of silver chloride which De composes when expos to light write the balanced equation.for the reaction
Answers
The decomposition reaction of silver chloride (AgCl) when exposed to light can be represented by the following balanced equation:
2AgCl (s) → 2Ag (s) + Cl2 (g)
In this equation, solid silver chloride decomposes into silver metal (Ag) and gaseous chlorine (Cl2) when exposed to light.
This reaction is an example of a photochemical reaction, where light energy triggers a chemical change. In this case, the absorption of light energy causes the silver chloride crystal lattice to break down, resulting in the formation of silver atoms and chlorine molecules.
It's worth noting that silver chloride is a photosensitive compound commonly used in traditional black and white photography. When light strikes the silver chloride-coated film, it creates a pattern of exposed and unexposed areas. The exposed areas undergo the decomposition reaction, resulting in the formation of metallic silver, which forms the photographic image.
For more such questions on silver chloride
https://brainly.com/question/19054138
#SPJ8
Lead ions can be removed from solution by precipitation with sulfate ions. Suppose a solution contains lead(II) nitrate.

Answers
1) List the compounds involved in the reaction.
Lead (II) nitrate
Potassium sulfate
Lead (II) sulfate
Potassium nitrate.
2) Write the formula of every compound.
Lead (II) nitrate: Pg(NO3)2
Potassium sulfate: K2SO4
Lead (II) sulfate: PbSO4
Potassium nitrate: KNO3
3) Write the chemical equation
.
\(Pb(NO_3)_2+K_2SO_4\rightarrow PbSO_4+KNO_3\)List the elements (or polyatomic ions) in the reactants.
Pb: 1
NO3: 2
K: 2
SO4: 1
List the elements (or polyatomic ions) in the products.
Pb: 1
NO3: 1
K: 1
SO4: 1
Balance K.
\(Pb(NO_3)_2+K_2SO_4\rightarrow PbSO_4+2KNO_3\)List the elements (or polyatomic ions) in the reactants.
Pb: 1
NO3: 2
K: 2
SO4: 1
List the elements (or polyatomic ions) in the products.
Pb: 1
NO3: 2
K: 2
SO4: 1
4) Balanced chemical equation.
\(Pb(NO_3)_{2(aq)}+K_2SO_{4(aq)}\rightarrow PbSO_{4(s)}+2KNO_{3(aq)}\).
In a sealed and rigid container, a sample of gas at 4.40 atm and 60.0
°C is cooled to 20.0 °C. What is the pressure (in atm) of the gas at
20.0 °C?
Answers
Explanation:
To find the pressure of the gas at 20.0 °C, we can use the combined gas law, which states:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 = Initial pressure
V1 = Initial volume
T1 = Initial temperature
P2 = Final pressure (what we're trying to find)
V2 = Final volume (assuming the volume remains constant)
T2 = Final temperature
Given:
P1 = 4.40 atm
T1 = 60.0 °C = 333.15 K (converting to Kelvin)
T2 = 20.0 °C = 293.15 K (converting to Kelvin)
Since the volume is assumed to remain constant (rigid container), we can simplify the equation as follows:
P1 / T1 = P2 / T2
Now, we can substitute the given values and solve for P2:
(4.40 atm) / (333.15 K) = P2 / (293.15 K)
Cross-multiplying:
P2 = (4.40 atm) * (293.15 K) / (333.15 K)
≈ 3.874 atm
Therefore, the pressure of the gas at 20.0 °C is approximately 3.874 atm.
In a sentence answer "Why do gases exert pressure on the walls of their container?"
Answers
Answer:
The pressure exerted by a gas is due to the random motion of particles in the gas. Gases have weak intermolecular forces and the particles are in continuous random motion and these particles collide with the walls of the container. These collisions with the walls of the container exert pressure on the gas.
Explanation: Hope this helps!!
The normal boiling point of ethanol is 78.4 oC. Its enthalpy of vaporization is 38.6 kJ/mol. Estimate the vapor pressure of ethanol at 26.3 oC.
Answers
Answer: The vapor pressure of ethanol at \(26.3^{o}C\) is 238.3 torr.
Explanation:
Given: \(\Delta H_{vap}\) = 38.6 kJ/mol
\(T_{1} = 26.3^{o}C = (26.3 + 273) K = 299.3 K\)
\(T_{2} = 78.4^{o}C = (78.4 + 273) K = 351.4 K\)
Formula used to calculate the vapor pressure of ethanol is as follows.
\(ln\frac{P_{2}}{P_{1}} = \frac{\Delta H_{vap}}{R} [\frac{1}{T_{1}} - \frac{1}{T_{2}}]\\\)
Substitute the values into above formula as follows.
\(ln\frac{P_{2}}{P_{1}} = \frac{\Delta H_{vap}}{R} [\frac{1}{T_{1}} - \frac{1}{T_{2}}]\\ \\ln \frac{760 torr}{P_{1}} = \frac{38600 J}{8.314 J/mol K}[\frac{1}{299.3} - \frac{1}{351.4}]\\\frac{760}{P_{1}} = 3.18\\P_{1} = 238.3 torr\)
Thus, we can conclude that the vapor pressure of ethanol at \(26.3^{o}C\) is 238.3 torr.
It was found that 2.35 g of a compound of phosphorus and chlorine contained 0.539 g of phosphorus. What are the percentages by mass of phosphorus and chlorine in this compound?
Answers
Answer:
Explanation:
P = 2.35g
Cl= 0.539 g
% MASS = mass of X/ mass of a compound
% mass of P = 2.35 / (2.35+ 0.539) = 81.34%
% mass of Cl = 0.539 /(2.35+ 0.539) = 18.66 %
Answer:
what the person above me said
Explanation:
16 grams of methane gas combined with 64 grams of oxygen to form 44 grams of carbon dioxide, plus water . What mass of water is produced ?
Answers
Answer:36 grams of water is produced. Law of conservation of matter states that mater cannot be created or destroyed. 16 grams of methane added to 64 grams of oxygen equals 80 total grams of reactants. 80 grams minus 44 grams of carbon dioxide is 36. This means there must be 36 grams of water, and you end with the same mass of reactants as in the beginning. Hope this helps! Good luck!
Explanation:
16 grams of methane gas combined with 64 grams of oxygen to form 44 grams of carbon dioxide, plus water. The mass of water is produced is 36 grams.
What is mass?Mass is defined as inertia, a fundamental feature of all stuff, is quantified.
It can also be defined as as the quantity of substance contained in any object or body.
Mass can be expressed as
Mass = volume x density
Mass = given mass / formula mass
The law of conservation of mass can be stated that the mass cannot be generated or destroyed, but can only be converted from one form to another.
Total mass = 64 + 16 = 80 grams
Mass of water = 80 - 44
= 36 grams
Thus, 16 grams of methane gas combined with 64 grams of oxygen to form 44 grams of carbon dioxide, plus water. The mass of water is produced is 36 grams.
To learn more about mass, refer to the link below:
https://brainly.com/question/19694949
#SPJ2
Name each of the following Acids and Bases:W(OH)5
Answers
The compound:
\(W(OH)_5\)Can be called as Tungsten (V) hydroxide.
A hypothesis does not need to be correct for an experiment to be successful because
a hypothesis is an educated guess about what will happen in an experiment.
True
Or
False
Answers
Pick an answer and explain why the others are incorrect.

Answers
The name of this compound using IUPAC rules is 3,4-dimethylhexane.
Option D is correct.
What are IUPAC rules?the IUPAC nomenclature of organic chemistry is described as a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry.
Option A, 2,3-diethylbutane, is incorrect because it has a different carbon chain length and different substituent positions.
Option B, 2-ethyl-3-methylpentane, is incorrect because it has a different carbon chain length and one of the substituents is incorrectly placed.
Option C, 3-methyl-4-ethylpentane, is incorrect because it has a different carbon chain length and the substituent positions are reversed.
Learn more about IUPAC rules at: https://brainly.com/question/28872356
#SPJ1
Which describes a possible path a carbon atom could take through the carbon cycle?
O A. Decomposers make coal into carbon. Carbon is released into the atmosphere. Plants take in carbon. Plant matter forms coal.
O B. Plants containing carbon die. Decomposers break down plant matter. Plant matter forms coal. Humans burn coal that releases
carbon into the atmosphere.
OC. Plant matter breaks down carbon in coal. Humans burn coal that releases carbon into the atmosphere. Decomposers break down
carbon in the atmosphere. Carbon in the atmosphere forms coal.
D. Humans burn coal that releases carbon into the atmosphere. Decomposers break down carbon in the atmosphere. Plant matter
breaks down carbon in the atmosphere. Plants containing carbon die.
Answers
Answer:
I know for a fact the correct answer is B
Answer:
A
Explanation:
BECAUSE plants use carbon dioxide for photosynthesis.
what type of bonds exists between neighboring water molecules?
Answers
Answer:
covalent bonds
Explanation:
which list of particles is in order of increasing mass
Answers
The correct order of particles in increasing mass is option A) Electron, neutron, proton
The electron, being the lightest particle, has the smallest mass among the three. It weighs approximately 9.1 x 10^-31 kilograms. Neutrons, slightly heavier, have a mass of around 1.67 x 10^-27 kilograms. Protons, being the heaviest, have a mass of about 1.67 x 10^-27 kilograms.
In conclusion, the order of particles in increasing mass is electron, neutron, and proton. The electron, with the smallest mass, is followed by the neutron, and the proton is the heaviest among the three particles.
To know more about Electron, neutron, proton click here:
https://brainly.com/question/29248303
#SPJ11
The complete question is:
Which list of particles is in order of increasing mass:
A) Electron, neutron, proton
B) Neutron, electron, proton
C) Proton, electron, neutron
What is importance of thermometer?
Answers
Courtney burns wood in an outdoor firepit. She measures the mass of the ashes that remain after the fire, and the mass is much lower than the wood that she burned. What would be the best explanation for what Courtney observes?
A. gases are released into the air
B. water inside the wood solidifies
C. heat caused the molecules to lose density
D. atoms in the wood are destroyed
Answers
Courtney burns wood in an outdoor firepit and she measures mass of the ashes that remain after the fire, and mass is much lower than the wood that she burned. The best explanation for what Courtney observes is that: A. gases are released into the air.
What is the best explanation for what Courtney observes?When wood burns, it undergoes chemical reaction called combustion, where it reacts with oxygen from the air to produce carbon dioxide, water vapor and other gases. These gases are released into air as the wood burns and their mass is not accounted for in ashes left behind.
Water inside the wood may also be released as steam during combustion process but this would not explain the significant difference in mass between the wood and ashes.
To know more about burning of wood, refer
https://brainly.com/question/1537286
#SPJ9
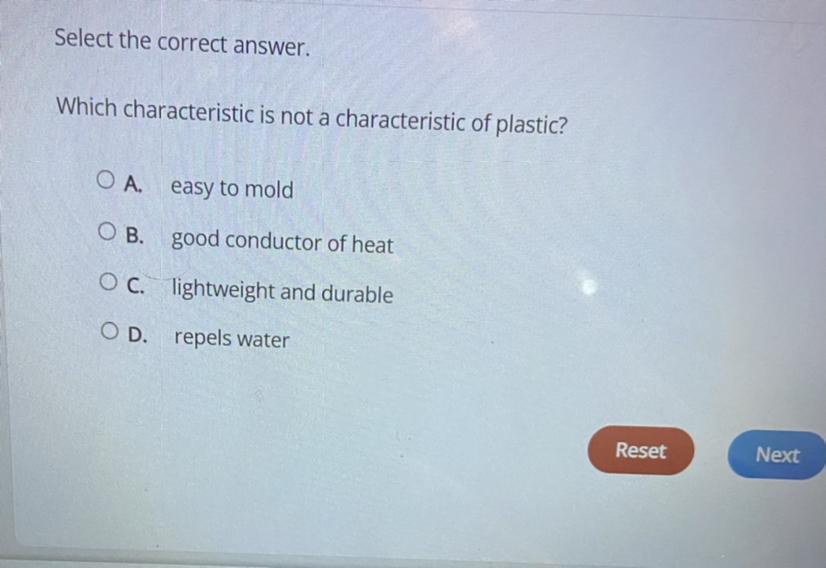
Which characteristic is not a characteristic of plastic?
A.) easy to mold
B.) good conductor of heat
C.) lightweight and durable
D.)Repels water
Image of question shown below
Answers
Answer:
I hope this helps
Explanation:

What happens to the molecules in the room as they change from a liquid to a gas?
Answers
When a liquid changes to a gas, the molecules in the room gain enough energy to break the bonds that hold them together in the liquid state.
What are Molecules?
Molecules are the smallest unit of matter that still has the properties of a particular substance. They are made up of two or more atoms that are bonded together. Molecules can be covalent, meaning the atoms in the molecule share electrons, or ionic, meaning one atom has donated electrons to the other.
This energy comes from an increase in temperature, which causes the molecules to move faster and farther apart. As the molecules move around and expand, they fill the room with a vapor or gas.
To know more about molecules,
https://brainly.com/question/26044300
#SPJ4
How many protons are in this atom if it has a balanced charge?
- 0
- 2
- 4
- 6

Answers
Answer:
6
Explanation:
If there are 6 electrons and it has a balanced charge, there also must be six protons.
The difference between sunspots solar flares and prominence
Answers
Answer:
Sunspots are cooler and darker than the rest of the Sun's surface. They are marked by intense magnetic activity. Solar prominences are the plasma loops that connect two sunspots. Solar flares and coronal mass ejections are eruptions of highly energetic particles from the Sun's surface.
Explanation:
Answer:
Sunspots are dark areas on the surface of the Sun that are cooler than surrounding areas. They appear in groups, vary in size, and cycle in number over an 11-year period. Solar flares are sudden eruptions of energy from a small area of the Sun's surface. They are extremely hot (10 to 20 million degrees Celsius) and extend into the corona. Solar flares occur near sunspots and can disturb radio communications on Earth.
Explanation: