calculate the freezing point and boiling point of a solution containing 8.20 g of ethylene glycol (c2h6o2) in 87.9 ml of ethanol. ethanol has a density of 0.789 g/cm3.
Answers
The freezing point of the solution is -117.787 C and Boiling point of the solution is 80.722 C.
A solution containing 8.20 g of ethylene glycol in 87.9 ml of ethanol. ethanol has a density of 0.789 g/cm3.
The mass of ethanol = density of ethanol * volume
= 0.789 * 87.9 ml = 69.353 g
molar mass of ethylene glycol = 62 g/mole
therefore moles of ethylene glycol in 8.20 g of it = 8.20/62 = 0.132 mole
molality = moles of ethylene glycol/mass of ethanol in kg
= 0.132 / 0.069353 = 1.9033 m
KF for ethanol = 1.99 C/m
KB for ethanol = 1.22 C/m
boiling point of ethanol = 78.4 C
The freezing point of a solution is less than the freezing point of the pure solvent. This means that a solution must be cooled to a lower temperature than the pure solvent in order for freezing to occur.
freezing point of ethanol = -114 C
depression in freezing point = molality * KF = 1.9033*1.99 = 3.787
thus freezing point of solution = -117.787 C
elevation in boiling point = molality * KB = 1.9033 * 1.22 = 2.3220
thus boiling point of solution = 80.722 C
To learn more about Freezing point and boiling point please visit:
https://brainly.com/question/40140
#SPJ4
Related Questions
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
What could you predict about the number of magnetic domains and the strength of a magnet
Answers
The strength of a magnet is affected by its size, material of construction, and the quantity of "tiny magnets" lined up inside the magnet. A larger magnet constructed of the same material .
What is the strongest magnet strength?Bar magnets are the weakest magnets because their poles are the weakest component. With a strength of up to 34 tons, a niobium magnet is the largest magnet known to man. When activated, the ferromagnetic materials magnetic field makes a shrill noise due to its extreme strength.
Does a stronger magnet have to be bigger?A balloon's Gauss value is influenced by both the material and geometry. For instance, if you have two magnets of different sizes made of the same material, but with differing surface Gauss values, the larger attraction are always stronger.
To know more about Strength of a magnet visit:
https://brainly.com/question/27850748
#SPJ4
In lab, you calculate the density of an iron rod to be 7.30 g/cm3. The accepted value
for the density of iron is 7.80 g/cm3. What is your percent error?
Answers
Answer:
6.41 %Explanation:
The percentage error of a certain measurement can be found by using the formula
\(P(\%) = \frac{error}{actual \: \: number} \times 100\% \\ \)
From the question
actual density = 7.80 g/cm³
error = 7.30 - 7.80 = 0.5
We have
\(p(\%) = \frac{0.5}{7.8} \times 100 \\ = 6.410256...\)
We have the final answer as
6.41 %Hope this helps you
the kind of friction that shows a falling object is it gravity, free fall
Answers
Answer:
It's called air resistance.
Explanation:
While it may seem hard to believe at first, all objects in free fall accelerate at the same rate regardless of their masses. Objects falling through air experience a type of fluid friction called air resistance.
Energy pyramid worksheet

Answers
Answer:
From top to bottom coyote, crow, squirrel, then acorn
Explanation:
The coyote has the least amount of energy and its the biggest predator so it belongs at the top. The crows eat squirrels and the squirrels eat acorns.
sodium hydrogen carbonate (nahco3) is often used to neutralize spills of acids such as sulfuric acid (h2so4). the neutralization reaction results in the production of h2o, co2, and na2so4(aq). what mass of co2 would be produced by the neutralization of 25.0 ml of 6.00m h2so4 using excess nahco3?
Answers
To determine the mass of CO2 produced by the neutralization of 25.0 mL of 6.00M H2SO4 using excess NaHCO3, we need to use stoichiometry.
The balanced chemical equation for the reaction is: H2SO4 + 2NaHCO3 → Na2SO4 + 2H2O + 2CO2
First, we need to convert the volume of H2SO4 to moles:25.0 mL × (1 L / 1000 mL) × (6.00 mol / 1 L) = 0.150 mol H2SO4
Next, we can use the mole ratio from the balanced equation to determine the moles of CO2 produced:0.150 mol H2SO4 × (2 mol CO2 / 1 mol H2SO4) = 0.300 mol CO2
Finally, we can convert the moles of CO2 to grams using the molar mass of CO2 (44.01 g/mol):0.300 mol CO2 × (44.01 g / 1 mol) = 13.2 g CO2
Therefore, the mass of CO2 produced by the neutralization of 25.0 mL of 6.00M H2SO4 using excess NaHCO3 is 13.2 g.
Know more about sodium hydrogen carbonate
https://brainly.com/question/2737841
#SPJ11
calculate the number of moles of nitrogen gas present in 400cm3 sample of it a pressure of 790 mmHg and 27°C.
Answers
Answer:
0.02 mol
Explanation:
Given data:
Number of moles of nitrogen = ?
Volume of sample = 400 cm³
Pressure = 790 mmHg
Temperature = 27°C
Solution:
Volume of sample = 400 cm³ (400/1000=0.4 L)
Pressure = 790 mmHg (790/760 = 1.04 atm)
Temperature = 27°C (27+273=300 K)
The given problem will be solve by using general gas equation,
PV = nRT
P= Pressure
V = volume
n = number of moles
R = general gas constant = 0.0821 atm.L/ mol.K
T = temperature in kelvin
1.04 atm ×0.4 L = n× 0.0821 atm.L/ mol.K ×300 K
0.416 atm.L = n× 24.63 atm.L/ mol
n = 0.416 atm.L /24.63 atm.L/ mol
n = 0.02 mol
take into account the speed of the top surface of the tank and find the speed of fluid leaving the opening at the bottom, if h=y2?y1, and A1 and A2 are the areas of the opening and of the top surface, respectively. Assume A1?A2 so that the flow remains nearly steady and laminar.
Express your answer in terms of the variables h, A1, A2, and appropriate constants.
Answers
The speed of the fluid leaving the opening at the bottom is approximately equal to v₂ ≈ (A₁v₁) / A₂
The speed of the fluid leaving the opening at the bottom can be determined using the principle of continuity, which states that the mass flow rate of a fluid remains constant in a steady and laminar flow.
According to the principle of continuity, the equation can be expressed as:
A₁v₁ = A₂v₂
Where A1 and A2 are the areas of the opening and the top surface respectively, v1 is the speed of the fluid at the top surface, and v2 is the speed of the fluid leaving the opening at the bottom.
Given that h = y₂ - y₁, where h is the height difference between the top surface and the opening, and assuming A₁ ≈ A₂, we can rewrite the equation as:
A₁v₁ ≈ A₂v₂
Now we can solve for v₂:
v₂ ≈ (A₁v₁) / A₂
Expressing the answer in terms of the given variables and appropriate constants, the speed of the fluid leaving the opening at the bottom is approximately equal to (A₁v₁) / A₂
To know more about fluid refer here:
https://brainly.com/question/6329574
#SPJ11
What is the power of a food processor that can perform 1,350 joules of work in 15 seconds
Answers
[M(CO)7]+ The 18 electron rule can also be used to help identify an unknown transition metal in a compound. Take for example [M(CO)7]+. To find what the unknown transition metal M is, simply work backwards: Example 24.3.3: [Co(CO)5]z Similarly to Example 2, the 18 electron rule can also be applied to determine the overall expected charge of an molecule. Take for example [Co(CO)5]x. To find the unknown charge z :
Answers
For the complex [Co(CO)5]x, the unknown charge (z) would be +1 based on the application of the 18 electron rule.
To find the charge (z) of the complex [Co(CO)5]x using the 18 electron rule, we can follow the steps below:
Identify the metal: In this case, the metal is cobalt (Co).
Determine the number of valence electrons of the metal: Cobalt is a transition metal with atomic number 27. In its neutral state, it has 27 electrons. However, in a complex, cobalt typically contributes all of its valence electrons to bonding, which is 9 electrons (2 from the 4s orbital and 7 from the 3d orbital).
Calculate the total number of electrons contributed by ligands: The ligand in this case is carbon monoxide (CO), which is a strong-field ligand. Each CO ligand contributes 2 electrons (one from the carbon lone pair and one from the oxygen lone pair) for a total of 5 ligands × 2 electrons/ligand = 10 electrons.
Add the valence electrons of the metal and the ligands: Cobalt contributes 9 electrons, and the CO ligands contribute 10 electrons, giving a total of 9 + 10 = 19 electrons.
Apply the 18 electron rule: According to the 18 electron rule, most stable transition metal complexes have 18 valence electrons. However, there can be variations depending on the ligands and the metal's oxidation state.
Determine the charge (z): Since the complex [Co(CO)5]x has 19 valence electrons, which is more than the expected 18 electrons, it suggests that the complex has a positive charge to balance the extra electron(s). Therefore, the charge (z) of the complex would be +1.
To know more about 18 electron rule
brainly.com/question/33426424
#SPJ11
plastics
shells
aluminum
cans
drift
wood
sea
weed
glass
This chart illustrates the type and number of items collected during a clean up of a
beach. Based on the chart, what approximate percentage of the total items result
from the action of people?
Answers
L.
Use a pencil and draw a line in the sequences below for
species A and species B to show where the catalyst
would cut the DNA.
Species A AATTGGCCTAATTAATTCGG CCTAG
Species B: AATTCCTACGG CCTAGCCTTTAATT
Answers
The catalyst BamH1 will cut the DNA as follows:
Species A: AATTG | GCCTAATTAATTCG | GCCTAG
Species B: AATTCCTACG | GCCTAGCCTTTAATT
What are restriction endonucleases?Restriction endonucleases or restriction enzymes are enzymes that cleave DNA into pieces at or close to particular recognition regions inside molecules called restriction sites.
EcoRI, BamH1, and smaI are some examples of restriction endonucleases.
BamHI is a type II restriction enzyme that recognizes the DNA sequence "GGATCC" and cuts the DNA at in between G and G.
Learn more about restriction endonucleases at: https://brainly.com/question/1127662
#SPJ1
Which of the following graphs correctly represents the relationship between the pressure and the volume of an ideal gas that is held at constant temperature
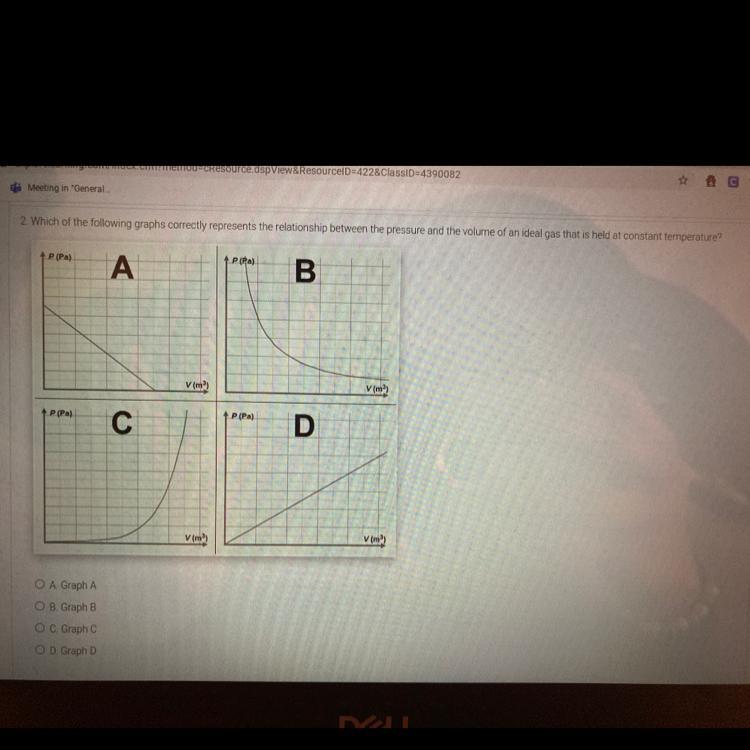
Answers
Answer:
B
Explanation:
Pressure is inversely proportional to temperature
the solution
. Which of the following trioxonitrate (v) will decomposes to its corresponding metal?
A. AgNO3 B. Zn(NO3)2 C. Pb(NO3)2 D. Cu(NO3)2
Answers
Answer:
A. AgNO3
I hope it's helps
how many atoms are there in 1.25 moles of Zn?
Answers
Answer:
7.53×10^23
Explanation:
i dont know how
Answer:
There are 6.25×10²³ atoms in 1.25 moles of Zn
Which of the following is not a gas in the Earth's atmosphere? A. oxygen B. carbon dioxide C. sulfur D.nitrogen 1
Answers
The Avogadro constant is 6.02 x 1023 mol-¹.
Calculate the number of moles of:
a water molecules, H₂O, in 9 g of water
Answers
The number of moles 18.01528.
How to find number of moles?
In the International System of Units, the mole is the unit of substance amount. A mole of a substance is defined as a mass of material that contains exactly 12,000 g of 12C's exact number of atoms as fundamental units. One mole has 600 sextillion molecules. While employing the mole, complicated calculations are more easily understandable. To get the number of moles, divide the compound's known mass by its molar mass. Consider a scenario where your sample of Na2SO4 weighs 20 g. 20 grammes divided by 142 grammes per mole yields 0.141 moles.
To know more about moles, refer: -
https://brainly.com/question/14276478
SPJ1
1.what is the VSEPR number for T - shape molecular geometry?
2. what is the molecular geometry for the VSEPR number 660?
3. what is the molecular Geometry for the VSEPR number 321?
Answers
1) The VSEPR number for T-shaped molecular geometry is 5.
2) The geometry is octahedral
3) The geometry is Trigonal pyramidal
What is the VSEPR?The VSEPR (Valence Shell Electron Pair Repulsion) theory is used to predict the molecular geometry of a molecule based on the arrangement of electron pairs around the central atom.
For a T-shaped molecular geometry, there are three bonding pairs of electrons and two non-bonding pairs of electrons around the central atom. Therefore, the VSEPR number for T-shaped molecular geometry is 5.
The VSEPR number is the sum of the number of atoms bonded to the central atom and the number of lone pairs of electrons on the central atom.
Learn more about VSEPR:https://brainly.com/question/29756299
#SPJ1
20 POINTS! please one question

Answers
Answer:
Most likely Spring or fall
Explanation:
Hope this helped:)
Sorry I'm late
2. What do you think may have happened to the "bad" chocolate bar? Do you think
it was made poorly? Or do you think something happened to the chocolate on the
way to the customer? Also, is there a way Charlotte can avoid this issue in the future?
Explain.
Answers
Answer:
2
Explanation:
when a substance goes directly from a solid state to a gas state as dry ice
Answers
Answer:
Sublimation
Explanation:
Sublimation refers to the process by which the change of matter takes place directly from solid to liquid state. The matter from the solid-state directly changes into the gaseous state without changing into the liquid state. More energy is required in this process. This is an endothermic reaction. Dry ice is the solid carbon dioxide sublimes in the air.
Titanium's corrosion resistance is so strong that even titanium with oxygen impurities does not show a reduction in corrosion resistance. a. True b. False.
Answers
The statement "Titanium's corrosion resistance is so strong that even titanium with oxygen impurities does not show a reduction in corrosion resistance" is true as Titanium is a highly corrosion-resistant metal, which is attributed to its ability to form a stable, protective oxide layer on its surface.
This layer acts as a barrier, preventing the penetration of corrosive agents and thus maintaining the metal's integrity.
Even when oxygen impurities are present in titanium, the corrosion resistance remains strong. This is because the impurities do not significantly affect the formation of the oxide layer, which is primarily responsible for the metal's resistance to corrosion. Additionally, the presence of oxygen can actually increase the strength and hardness of titanium, further contributing to its durability.
In summary, titanium's corrosion resistance remains strong even with the presence of oxygen impurities, due to the protective oxide layer that forms on its surface and the potential for increased strength and hardness.
To know more about Titanium, refer here:
https://brainly.com/question/31605433#
#SPJ11
Question: Rank the following radicals in order of increasing stability.
Answer
The rank of the given radicals in the increasing order of stability is as follows:
Answers
Consider the following techniques to improve stability: mproving the foundation: Make certain that the foundation is strong, stable, and capable of sustaining the structure.
Maintaining balance: Keep the structure's weight equally distributed and balanced. Stress reduction: Avoid overstressing the structure and minimize current stress by suitable support and reinforcement. Material updates: Use materials that are recognized to be long-lasting and durable. Regular maintenance entails inspecting and maintaining the structure on a regular basis in order to discover and treat any concerns before they become big difficulties. Reduce dynamic loads: Keep vibrations and other dynamic loads to a minimum. Using suitable design: Ensure that the design takes into account the structure's stability needs. This is not a complete list, and the best method for boosting.
learn more about stability here:
https://brainly.com/question/30244458
#SPJ4
The patient is to receive potassium chloride 40mEq orally. The label states, "Potassium Chloride, 20mEq per 15ml. What volume (ml) will you administer?
Answers
You will need to administer 30ml of potassium chloride to the patient.
To calculate the volume (ml) of potassium chloride to administer, we can use a proportion. The given label states that there are 20mEq of potassium chloride in 15ml.
Let's set up the proportion:
20mEq / 15ml = 40mEq / x ml
To solve for x, cross-multiply:
20mEq * x ml = 15ml * 40mEq
Now divide both sides by 20mEq:
x ml = (15ml * 40mEq) / 20mEq
Simplifying further, we get:
x ml = 30ml
Learn more about potassium chloride from the given link
https://brainly.com/question/31563020
#SPJ11
what are true about bonds

Answers
Answer:
a.
Explanation:
The electrolysis of water forms h2 and o2. 2h2o right arrow. 2h2 o2 what is the percent yield of o2 if 10.2 g of o2 is produced from the decomposition of 17.0 g of h2o? use percent yield equals startfraction actual yield over theoretical yield endfraction times 100.. 15.1% 33.8% 60.1% 67.6%
Answers
The percent yield of oxygen if 10.2 g of O₂ is produced from the decomposition of 17.0 g of H₂O is 67.6%.
How do we calculate moles from mass?Moles (n) of any substance can be calculated by using their mass as:
n = W/M, where
W = given mass
M = molar mass
Given chemical reaction is:
2H₂O → 2H₂ + O₂
Moles of 17g H₂O = 17g / 18.02 g/mol = 0.943 moles
From the stoichiometry of the reaction it is clear that,
2 moles of H₂O = produces 1 mole of O₂
0.943 moles of H₂O = produces 1/2×0.943=0.471 mole of O₂
Now mass of this 0.471 moles of O₂ = (0.472mol)(32g/mol) = 15.104 grams
Actual mass of oxygen = 10.2g (given)
Now on putting vales on the percent yield formula, we get
% yield = (10.2 / 15.104)×100% = 67.53% = 67.6%
Hence the required value of percent yield is 67.6%.
To know more about percent yield, visit the below link:
https://brainly.com/question/25996347
Answer:67.6
Explanation:bc im just that good
The vapor pressure of the solvent above a solution is directly proportional to:__.
1. the molality of the solute in the solution.
2. the molarity of the solute in the solution.
3. the temperature in degrees Celsius.
4. the mole fraction of the solvent in the solution.
5. the temperature in Kelvins.
6. the molality of the solvent in the solution.
7. the mole fraction of the solute in the solution.
8. the molarity of the solvent in the solution.
Answers
The correct option among the given options is option 4, "the mole fraction of the solvent in the solution".
Raoult's law is a law that explains the relation between the vapor pressure of the solution and the mole fraction of the components present in the solution. It states that the partial vapor pressure of each component in the solution is equal to the product of its mole fraction and the vapor pressure of the pure component. Mathematically, this law can be expressed as:
P_A = X_A P^0_A
where, P_A is the partial vapor pressure of the solvent A above the solution, X_A is the mole fraction of the solvent A in the solution, and P^0_A is the vapor pressure of the pure solvent A.
When a non-volatile solute is added to a solvent, the mole fraction of the solvent decreases and the mole fraction of the solute increases. According to Raoult's law, the partial vapor pressure of the solvent decreases as its mole fraction decreases, and the partial vapor pressure of the solute is negligible as it is non-volatile. Therefore, the vapor pressure of the solution is less than the vapor pressure of the pure solvent. The lowering of the vapor pressure is directly proportional to the mole fraction of the solvent.
Therefore, the vapor pressure of the solvent above a solution is directly proportional to the mole fraction of the solvent in the solution.
Learn more about Raoult's law visit:
brainly.com/question/2253962
#SPJ11
calculate the frequency of light associated with the transition from n=2 to n=3
Answers
The frequency of light associated with the transition from n=2 to n=3 is approximately 5/36 times the Rydberg constant.The frequency of light associated with the transition from one energy level to another can be calculated using the Rydberg formula, which is given by:
ν = R * (1/n₁² - 1/n₂²)
where ν is the frequency of light, R is the Rydberg constant (approximately 3.29 x 10^15 Hz), n₁ is the initial energy level, and n₂ is the final energy level.
Given that the transition is from n=2 to n=3, we can substitute these values into the formula and calculate the frequency:
ν = R * (1/2² - 1/3²)
ν = R * (1/4 - 1/9)
ν = R * (9/36 - 4/36)
ν = R * (5/36)
The frequency of light associated with the transition from n=2 to n=3 is approximately 5/36 times the Rydberg constant.
To learn more about Rydberg constant click here: brainly.com/question/28168267
#SPJ11
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
When temperature drops, (for example from 20 degrees celsius to 10 degrees celsius)
a.) energy increases
b.) particles move slower
c.) particles collide more often
d.) pressure increases