Calculate each of the following quantities:
(b) Molarity of a solution that contains 7.25 mg of calcium chloride in each milliliter
Answers
Molarity of a solution that contains 7.25 mg of calcium chloride in each milliliter.
What is molarity?Molar concentration (also known as molarity, quantity concentration, or substance concentration) is a measure of the concentration of a chemical species in a solution, specifically of a solute, in terms of amount of substance per unit volume of solution. The most often used unit for molarity in chemistry is the number of moles per liter, denoted by the unit symbol mol/L or mol/dm3 in SI units. A solution with a concentration of 1 mol/L is referred to as 1 molar, or 1 M.
To learn more about molarity visit:
https://brainly.com/question/8732513
#SPJ4
Related Questions
Una definición adecuada para el estado plasma sería:
Answers
Plasma is a state of matter in which a gas has been ionized to the point where it contains a significant number of free electrons and positive ions.
In a plasma, the electrons are separated from their parent atoms or molecules and are free to move about. This creates a mixture of positively charged ions and negatively charged electrons, which collectively behave like a fluid rather than individual particles.
Plasmas can be created by heating a gas to high temperatures, subjecting it to a strong electromagnetic field, or by passing an electric current through it. Examples of natural plasmas include lightning, the aurora borealis, and the sun. Plasmas have unique properties and are used in a variety of applications, including fluorescent lighting, plasma cutting and welding, and in plasma TVs.
To know more about the Plasma, here
https://brainly.com/question/954239
#SPJ4
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Write the first five members of Alkanes
Answers
Ethane (C2H6)
Propane (C3H8)
Butane (C4H10)
Pentane (C5H12)
After adding the sulfuric acid and letting that reaction proceed to completion, what chemical species were left in the beaker?.
Answers
After adding the sulfuric acid and letting that reaction proceed to completion, Copper sulfate and water chemical species were left in the beaker.
With the chemical formula H2SO4, sulfuric acid, sometimes referred to as oil of vitriol in antiquity, is a mineral acid made up of the elements hydrogen, oxygen, and sulphur. It is a viscous liquid that is miscible with water and is colourless, odourless, and viscous. Because of its strong affinity for water vapour and because it is hygroscopic and rapidly collects water vapour from the air, pure sulfuric acid does not occur naturally on Earth. Due to its potent dehydrating and oxidising qualities, concentrated sulfuric acid is extremely corrosive to various materials, including rocks and metals.
In the industrial setting, copper sulphate is made by heating concentrated sulfuric acid or copper oxides and treating them with the acid. Copper sulphate is typically bought for use in laboratories.
learn more about sulfuric acid here
https://brainly.com/question/1107054
#SPJ4
a saturated solution of magnesium fluoride in water at 20o c contains 0.130 g of solute per liter of solution. what is the concentration of magnesium ions in this solution?
Answers
The concentration of magnesium ions in the saturated solution of magnesium fluoride is 0.00208 mol/L.
To determine the concentration of magnesium ions in the solution, we need to understand the dissociation of magnesium fluoride (MgF₂) in water.
MgF₂ dissociates into one magnesium ion (Mg²⁺) and two fluoride ions (F⁻) in water, according to the balanced chemical equation:
MgF₂(s) ↔ Mg²⁺(aq) + 2F⁻(aq)
Since magnesium fluoride is a strong electrolyte, it completely dissociates in water. Therefore, the concentration of magnesium ions in the solution will be equal to the concentration of MgF₂.
Given that the saturated solution of magnesium fluoride contains 0.130 g of solute per liter of solution, we can use this information to calculate the concentration of magnesium ions.
The molar mass of MgF₂ is:
Molar mass of MgF₂ = atomic mass of Mg + 2 × atomic mass of F
= 24.31 g/mol + 2 × 18.99 g/mol
= 62.29 g/mol
Now, we can calculate the concentration of magnesium ions (Mg²⁺) in the solution using the following formula:
Concentration (in mol/L) = (mass of solute) / (molar mass of solute)
Concentration of Mg²⁺ = Concentration of MgF₂ = (0.130 g/L) / (62.29 g/mol)
Calculating the concentration:
Concentration of Mg²⁺ = 0.130 g/L / 62.29 g/mol = 0.00208 mol/L
Learn more about the saturated solution at https://brainly.com/question/1851822
#SPJ11
What is the correct term for a community of plants and animals and their physical surroundings?
individuals (organism)
continent
territory
ecosystem
Answers
predict the major organic product formed on thermal decarboxylation of the following compound.
Answers
The compound given is 3-methyl-2-pentenoic acid.
Thermal decarboxylation of 3-methyl-2-pentenoic acid is a reaction in which the carboxylic acid group is removed from the molecule, resulting in the formation of an alkene.
In this reaction, the acid group is first protonated and then the bond between the alpha carbon and the carboxyl oxygen is broken. The major organic product formed on thermal decarboxylation of 3-methyl-2-pentenoic acid is 2-methyl-1-pentene.
The initial step of the reaction involves the protonation of the carboxylic acid group of 3-methyl-2-pentenoic acid by a strong acid, typically sulfuric acid. This protonation activates the carboxyl group and makes it more susceptible to decarboxylation.
The subsequent step involves the breaking of the bond between the alpha carbon and the carboxyl oxygen, resulting in the loss of carbon dioxide and the formation of the alkene. The formed alkene in this reaction is 2-methyl-1-pentene.
Therefore, the major organic product formed on thermal decarboxylation of 3-methyl-2-pentenoic acid is 2-methyl-1-pentene.
Know more about decarboxylation here
https://brainly.com/question/17312252#
#SPJ11
Iron has a density of 7.9 g/cm3. What is the mass of a cube of iron with the length of one side equal to 55.0 mm?
Question 3 options:
1.3 × 103 g
2.3 × 10-2 g
4.3 × 102 g
2.1 × 104 g
1.4 g
Answers
The mass of the cube of iron with a side length of 55.0 mm is approximately 1313.6125 grams.
To calculate the mass of a cube of iron, we need to know the density of iron and the length of one side of the cube. Given that the density of iron is 7.9 g/cm^3 and the length of one side of the cube is 55.0 mm, we can proceed with the calculation. First, we need to convert the length of one side from millimeters (mm) to centimeters (cm) since the density is given in grams per cubic centimeter. We divide 55.0 mm by 10 to obtain 5.5 cm.
Next, we can calculate the volume of the cube using the formula V = (side length)^3. Substituting the value of 5.5 cm into the formula, we get V = (5.5 cm)^3 = 166.375 cm^3. Finally, we can calculate the mass of the cube using the formula mass = density × volume. Substituting the values of density (7.9 g/cm^3) and volume (166.375 cm^3), we get mass = 7.9 g/cm^3 × 166.375 cm^3 = 1313.6125 g.
In summary, to calculate the mass of the iron cube, we convert the length from millimeters to centimeters, calculate the volume of the cube, and then multiply it by the density of iron. The resulting mass is approximately 1313.6125 grams.
To learn more about formula mass click here:
brainly.com/question/28647347
#SPJ11
determine how many carbon dioxide (co2) molecules are produced if 8.45 x 1023 molecules of water (h2o) are produced
Answers
By balancing the equation the ratio between CO₂ and H₂O is 4:6. By calculating we get the number of molecules of Carbon dioxide produced is 5.63 ×10²³.
The reaction here is the Ethane and oxygen reacts to form Carbon dioxide and water. The reaction is
C₂H₆ + O₂ -----------> CO₂ + H₂O
We can balance the reaction
2C₂H₆ + 7O₂ ----------> 4CO₂ + 6H₂O
2 moles of ethane reacts with 7 moles of oxygen to get 4 moles of carbon dioxide and 6 moles of water.
Here 8.45× 10²³ molecules of water is produced
To find the number of moles, use the equation,
Number of moles = Number of molecules / Avogadro's number
= 8.45× 10²³/ 6.022× 10²³
= 1.403 moles
Ratio between CO₂ and H₂O is 4:6. Using proportions the moles of CO₂ is \(\frac{4}{6} = \frac{x}{1.403}\)
x = 1.403 × 4/6 = 0.935 moles
Number of molecules in 0.935 moles of Carbon dioxide = 0.935 × 6.022×10²³ = 5.63 ×10²³
So, the answer is 5.63×10²³
For more information regarding number of moles and molecules, kindly refer
https://brainly.com/question/12513822
#SPJ4
Complete question is given as the image.

patterns of reactivity quick check
Answers
Reactivity decreases as you move from left to right. The farther to the left and down the periodic chart you move, easier it is for electrons to be given or taken away, hence resulting in higher reactivity.
What is the pattern of reactivity in periodic table?Reactivity decreases as we move down the column. As you learn more about the table, you will be able to find that this pattern is true for other families.
The atoms get bigger, as the atomic number increases. The chemical properties change slightly when compared to the element right above them on the table. The non-metal elements in Group 7 that are known as the halogens, get less reactive as you move to the down of the group.
To know more about pattern of reactivity, refer
https://brainly.com/question/6759644
#SPJ4
16) What is the aluminum ion concentration in a solution that is 0.646 M in aluminum sulfate
Answers
Aluminum ion has a charge of 3+, Al³⁺, and sulfate is SO₄²⁻, so the compound aluminum sulfate has to have a number of aluminum and sulfate such that the final charge is zero, so the proportion on aluminum sulfate is:
\(Al_2(SO_4)_3\)That way we have 6+ and 6-, so neutral compound.
This means that for 1 mol of aluminum sulfate, we have 2 moles of aluminum ion. The molar concentration is the number of moles of solute divided by the volume of solution, so it is directly proportional to the number of moles.
So, we can use a rule of three as follows:
aluminum ion --- aluminum sulfate
2 --- 1
x --- 0.646 M
So:
\(\begin{gathered} \frac{2}{x}=\frac{1}{0.646M} \\ 2\cdot0.646M=x\cdot1 \\ 1.292M=x \\ x=1.292M \end{gathered}\)So, the concentration of aluminum ion in this solution is 1.292 M.
If the reactants on the left side of a chemical equation are c3h8+ 5o2, what could the products in a balanced equation be ?.
Answers
The products of the reaction that will balance out the equation are carbon dioxide and water.
The reactants of the left side of the chemical equation are given to be Oxygen and C₃H₈.
C₃H₈ is a hydrocarbon which on combustion with oxygen will give carbon dioxide gas and water as the product.
Now we can see you from the above information that we are provided with 5 moles of oxygen molecule.
So, writing the complete balanced chemical equation,
C₃H₈ + 7/2O₂ → 3CO₂ + 4H₂0
The above is the required balanced chemical equation of the reaction.
To know more about chemical equation, visit,
https://brainly.com/question/11231920
#SPJ4
What is oxidized in a galvanic cell made with silver and nickel electrodes?
A. The silver metal
B. The nickel metal
C. The nickel ions
D. The silver ions
Answers
Answer:
B. The nickle Metal
Explanation:
A P E X
Geothermal energy is produced from a combination of high pressure and the breakdown of______ underground.
Fill in the blank
Answers
the diagrams above represent two allotropes of solid phosphorus. which of the following correctly identifies the allotrope with the higher melting point and explains why?
Answers
Allotrope 1 has the higher melting point because it lacks the covalent bonds between phosphorus atoms that are present in allotrope 2.
The Melting Point Differences Between Allotropes of Solid PhosphorusAllotropes of solid phosphorus are different forms of the element which differ in their molecular arrangement and structure. Allotrope 1 is a more stable form and has a higher melting point than that of allotrope 2 due to the absence of covalent bonds between the phosphorus atoms in allotrope 1.
The structure of allotrope 1 is an ordered arrangement in which the phosphorus atoms form a three-dimensional lattice. This lattice structure is held together by strong Van der Waals forces, which are electrostatic attractions between the atoms. This structure is more stable than that of allotrope 2 and has a higher melting point due to the increased strength of the interatomic forces.
In contrast, the structure of allotrope 2 is much less ordered, and the phosphorus atoms are held together by covalent bonds. This structure is not as stable as that of allotrope 1 and has a lower melting point. The covalent bonds between the phosphorus atoms are much weaker than the forces in allotrope 1, and consequently the melting point of allotrope 2 is lower.
This question is incomplete, so I am attaching the image that contains the information needed to answer it.
Learn more about Allotropes :
https://brainly.com/question/13058829
#SPJ4

8. Which has greater thermal energy: a glass of water
or a lake full of water? Why?
Answers
Answer:
Im assuming a glass of water
Explanation:
there is less water that needs to be heated up
1
Select the correct answer.
Which type of energy is thermal energy a form of?
OA.
chemical energy
B.
kinetic energy
O c.
magnetic energy
OD
potential energy
Reset
Next
Answers
Answer:
i think kinetic energy sorry if I'm wrong.
PLEASEEEE HELPPPP I’ll make you Brainlys Summary
Now, create of summary of what you've learned.
1. Complete the table below for protons, neutrons, and electrons. Respond with a Y for yes or N of no.
1
2
3
***
***
***
Question
Score: 0/4
Identifies Element?
Affects Mass?
Affects Charge?
***
Proton
***
Neutron
***
Electron
***

Answers
Answer:
Explanation:
For the first row, only protons identify an element. For the second row, protons and neutrons affect mass. For the third row, protons and electrons affect charge.
how is a gas affected when pressure temperature or volume change
Answers
Answer:
The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law).
Explanation:
Answer:
The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law).
Explanation:
Please help I will give brainly.
Thank you so much
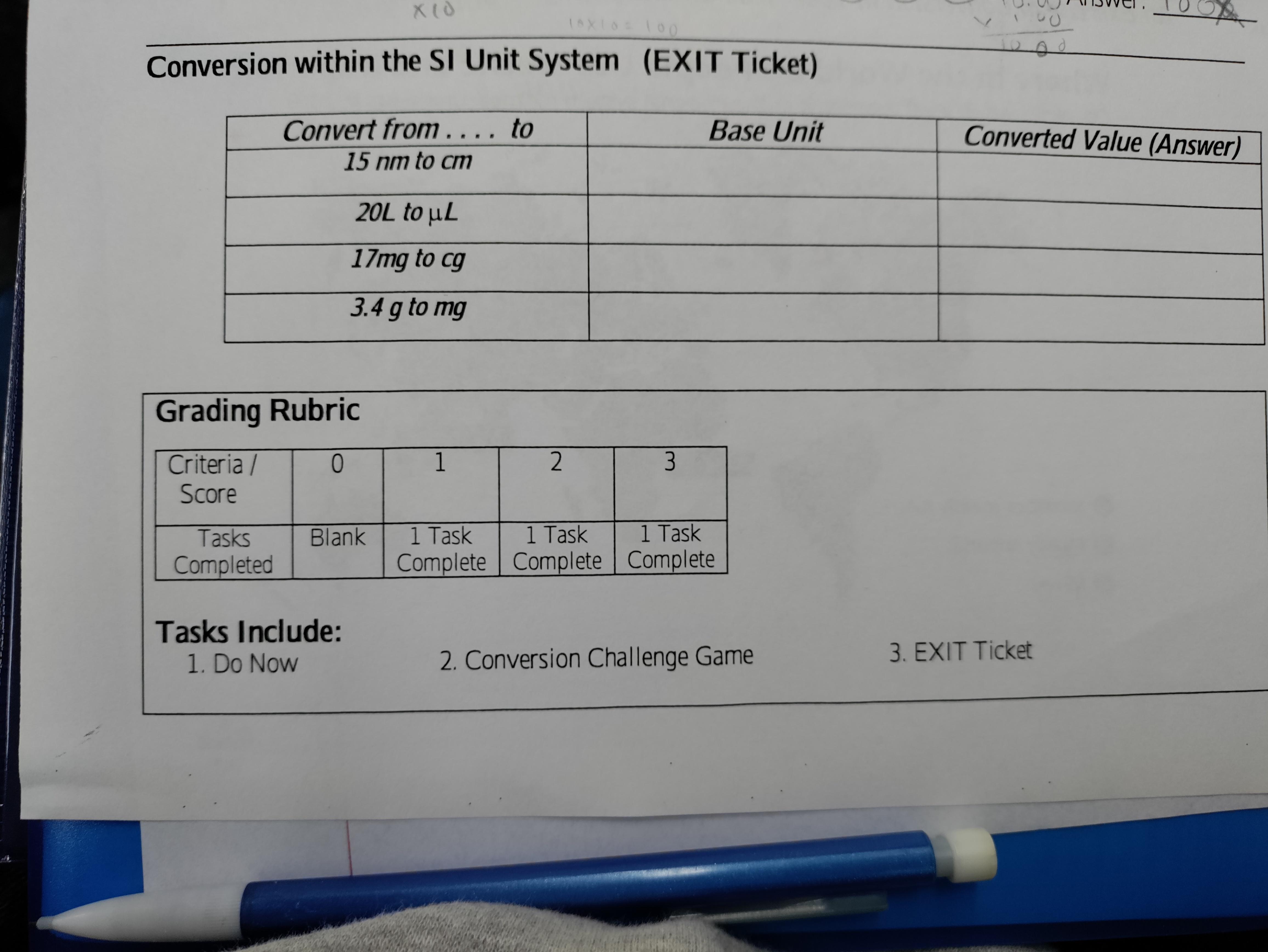
Answers
Answer:
meter. 1.5×10^-6
Litter. 2×10^-5
Kilo Gram. 1.7
Kilo Gram. 3.4 ×10^-3
I hope you understood that and if you don't understand write in comments
mass of 2 into 10 to power 21 number of atoms of an element is 0.4 gram what is the mass of 0.5 mole of the elements
Answers
The mass of 0.5 mole of the element is approximately 6.025 grams.
To calculate the mass of 0.5 mole of the element, we need to know the molar mass of the element.
Given that the mass of 2 x 10^21 atoms of the element is 0.4 grams, we can use this information to find the molar mass.
The number of atoms in 1 mole of any substance is given by Avogadro's number, which is approximately 6.022 x 10^23 atoms/mol.
First, we calculate the molar mass of the element using the given information:
Molar mass = Mass of 2 x 10^21 atoms / Number of moles of 2 x 10^21 atoms
Molar mass = 0.4 g / (2 x 10^21 atoms / (6.022 x 10^23 atoms/mol))
Molar mass ≈ 0.4 g / (3.32 x 10^-2 mol)
Molar mass ≈ 12.05 g/mol
Now that we know the molar mass of the element is approximately 12.05 g/mol, we can calculate the mass of 0.5 mole of the element:
Mass = Molar mass x Number of moles
Mass = 12.05 g/mol x 0.5 mol
Mass = 6.025 grams
for more such questions on element
https://brainly.com/question/28376204
#SPJ8
a 20.0-ml sample of hydrochloric acid (hcl) is titrated and found to react with 42.6 ml of 0.100 m naoh. what is the molarity of the hydrochloric acid solution?
Answers
The molarity of the hydrochloric acid solution in the reaction between NaOH and HCl, 1 mole of NaOH reacts with 1 mole of HCl. 1 NaOH + 1 HCl → 1 NaCl + 1 H2O (Mole ratio of NaOH to HCl is 1:1) • The concentration of the NaOH was 0.1 M, or 0.1 moles/liter.
What is molarity?
Molarity (M) is the quantity of a substance in a given extent of solution. Molarity is described because the quantity of moles of solute consistent with liter of solution. Molarity is likewise known as the molarity of a solution.
Therefore, The molarity of the hydrochloric acid solution in the reaction between NaOH and HCl, 1 mole of NaOH reacts with 1 mole of HCl. 1 NaOH + 1 HCl → 1 NaCl + 1 H2O (Mole ratio of NaOH to HCl is 1:1) • The concentration of the NaOH was 0.1 M, or 0.1 moles/liter.
To learn more about molarity refer the given link:-
https://brainly.com/question/14591804
#SPJ4
in figure 21-4b in what direction is the force defeating the wire
Answers
Answer:
the one that has more power to it
What are the groupings of the periodic table?
Answers
Answer:
The synopsis and according to particular circumstances is summed up in the description section below.
Explanation:
The periodic table would be a tabular configuration of such chemical elements. It's organized throughout order to increase the proportion of nuclear weapons. This same description is given to either the groupings mostly on the periodic table which are already decided to make up of components that seem to have specific chemical resistant inclinations as well as binding energies.
Why is large-scale nuclear fission a rare phenomenon on Earth?
Answers
Answer:
Large-scale nuclear is fission a rare phenomenon on Earth because
atoms that undergo fission aren't concentrated enough to sustain a chain reaction.
Hope it helps you.Answer:
Atoms that undergo fission aren't concentrated enough to sustain a chain reaction. For example, the decay chain of U-238 is called the uranium series. ... Uranium is find in ores on Earth with very law mass percentage in it, so fission is not possible
What volume in liters, l, of solution should sven prepare if he wants to make a 5.00 m solution using 210.0 grams, g, of sodium chloride, nacl? the molar mass of nacl is 58.44
Answers
To make a 5.00 M solution using 210.0 grams, g, of sodium chloride, NaCl, we will use the formula of volume of solution in liters is equal to mole of solute per molarity of solution, which is 0.718 L.
Molarity (M) refers to the amount of a substance in a specific volume of solution. It is defined as the moles of a solute per liters of a solution. Molarity is also known as the molar concentration of a solution.
Molarity is given by the quotient,
Molarity = moles of solute/volume of solution in liters.
Now in order to get the molarity of the solution, we have to divide the moles of solute over the liters of solution. Since we are given the mass of the solute, we must first convert the mass of NaCl into moles with the help of the molar mass of NaCl as a conversion factor.
210 NaCl x ( 1 mol NaCl/ 58.44g NaCl) = 3.59 mol NaCl.
We already have the molarity of the solution that is 5.00 M. So the solution to find out the volume will be,
Volume of solution in liters = mole of solute/ molarity of solution,
That is 3.59 mol NaCl / 5M = 0.718 L
To learn more about Molarity, head here
brainly.com/question/8732513
#SPJ4
What is the quantity of heat (in kJ) associated with cooling 185.5 g of water from 25.60°C to ice at -10.70°C?Heat Capacity of Solid = 2.092 J/g°CHeat Capacity of Liquid = 4.184 J/g°CT Fusion = 0.00 °CΔH Fusion = 6.01 kJ/mol
Answers
Taking into account the definition of calorimetry, sensible heat and latent heat, the amount of heat required is 37.88 kJ.
CalorimetryCalorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heatSensible heat is defined as the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
Latent heatLatent heat is defined as the energy required by a quantity of substance to change state.
When this change consists of changing from a solid to a liquid phase, it is called heat of fusion and when the change occurs from a liquid to a gaseous state, it is called heat of vaporization.
25.60 °C to 0 °CFirst of all, you should know that the freezing point of water is 0°C. That is, at 0°C, water freezes and turns into ice.
So, you must lower the temperature from 25.60°C (in liquid state) to 0°C, in order to supply heat without changing state (sensible heat).
The amount of heat a body receives or transmits is determined by:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
c= Heat Capacity of Liquid= 4.184 \(\frac{J}{gC}\)m= 185.5 gΔT= Tfinal - Tinitial= 0 °C - 25.60 °C= - 25.6 °CReplacing:
Q1= 4.184 \(\frac{J}{gC}\)× 185.5 g× (- 25.6 °C)
Solving:
Q1= -19,868.98 J
Change of stateThe heat Q that is necessary to provide for a mass m of a certain substance to change phase is equal to
Q = m×L
where L is called the latent heat of the substance and depends on the type of phase change.
In this case, you know:
n= 185.5 grams× \(\frac{1mol}{18 grams}\)= 10.30 moles, where 18 \(\frac{g}{mol}\) is the molar mass of water, that is, the amount of mass that a substance contains in one mole.
ΔHfus= 6.01 \(\frac{kJ}{mol}\)
Replacing:
Q2= 10.30 moles×6.01 \(\frac{kJ}{mol}\)
Solving:
Q2=61.903 kJ= 61,903 J
0 °C to -10.70 °CSimilar to sensible heat previously calculated, you know:
c = Heat Capacity of Solid = 2.092 \(\frac{J}{gC}\)m= 185.5 gΔT= Tfinal - Tinitial= -10.70 °C - 0 °C= -10.70 °CReplacing:
Q3= 2.092 \(\frac{J}{gC}\) × 185.5 g× (-10.70) °C
Solving:
Q3= -4,152.3062 J
Total heat requiredThe total heat required is calculated as:
Total heat required= Q1 + Q2 +Q3
Total heat required=-19,868.98 J + 61,903 J -4,152.3062 J
Total heat required= 37,881.7138 J= 37.8817138 kJ= 37.88 kJ
In summary, the amount of heat required is 37.88 kJ.
Learn more about calorimetry:
brainly.com/question/14057615?referrer=searchResults
brainly.com/question/24988785?referrer=searchResults
brainly.com/question/21315372?referrer=searchResults
brainly.com/question/13959344?referrer=searchResults
brainly.com/question/14309811?referrer=searchResults
brainly.com/question/23578297?referrer=searchResults
If a compound begins with a metal, it most likely is a _______ compound
metallic
molecular
covalent
ionic
Answers
Answer:
A
Explanation:
If a compound begins with metal, it is most likely to be a metallic compound. Thus, option A is correct.
The metallic compound has been formed when the metal has been bonded with a metal or more than 1 metal by the ionic or covalent interaction.
The ionic interactions result in between the metal and non-metal or between two metals, and the covalent interactions have been found in the nonmetals.
The molecular compound has been the one that has been formed a molecule. All the compounds are molecular compounds.
Thus, if it starts with metal, it has been most likely to form a metallic compound. Thus, option A is correct.
For more information about the compounds with metal, refer to the link:
https://brainly.com/question/18665430
Which plate is the South American plate?
Web
D
O A D
B. A
C. B
В
D C
Answers
Answer:
C. B
Explanation:
The correct plate is showing n the green color that is of South America and is a major tectonic plate. The plate belongs to the south American continent and forms the southernmost part of the mid-Atlantic ridge. According to the plate movement, it's still moving to the west away from the Atlantic ridges due to the seafloor spreading. Has The plate has an approx area of 43,600,000 km square.PLEASE ANSWER THIS FAST
Upper H Subscript 2 Baseline Upper S Upper O Subscript 4 Baseline (a q) double-headed arrow 2 Upper H Superscript + Baseline (a q) + Upper S Upper O Superscript 2 negative sign Subscript 4 Baseline (a q)
Which contain a common ion that will shift the equilibrium system represented by the equation shown? Select all that apply.
MgSO4
Na2S
HNO3
CaCl2
Answers
MgSO₄ and HNO₃ contain a common ion that will shift the equilibrium system. Therefore, option A, C is correct.
What is equilibrium?The equilibrium of a system can be defined as the state in which both the reactants and products in the chemical reactions are in concentrations that have not changed with time. The rates of reaction of the forward reactions and backward reactions are non-zero, but equal while in an equilibrium system.
By definition, a common ion can be described as an ion that enters the solution from two different sources. Solutions to which NaCl and AgCl are added also contain a common ion will be chloride Cl⁻ ion. The effect of common ions on solubility product equilibrium of the system.
The dissociation of sulphuric acid can be shown as:
H₂SO₄ → 2 H⁺ (aq) + SO₄²⁻ (aq)
MgSO₄ and H₂SO₄ have a common ion SO₄²⁻. H₂SO₄ and HNO₃ have a common ion H⁺ ion.
Learn more about equilibrium, here:
brainly.com/question/15118952
#SPJ2
Answer:
1. A, C
2. B- Equilibrium will sift to the left.
Explanation: