Calculate each of the following quantities:
(b) Amount (mol) of compound in 15.8 kg of Fe(ClO₄)₃
Answers
The amount (mol) of the compound in 15.8 kg of Fe(ClO₄)₃ is 0.044 moles.
What is a mole?The mole is the smallest unit of the atom which use to represent how many atoms moles or molecules are used in a chemical process or in a chemical reaction which is always equal to 2.303 ×10²³ moles for one atom.
To calculate the mass of 0.68 mol of KMnO₄ the formula will use is,
number of moles = mass / molar mass
molar mass of Fe(ClO₄)₃ = 354.19
mass of Fe(ClO₄)₃ = 15.8kg
substituting the value in the equation,
number of moles = 15.8kg / 354.19
number of moles = 0.044 moles
Therefore, 0.044 moles is the amount (mol) of the compound in 15.8 kg of Fe(ClO₄)₃.
Learn more about moles, here:
https://brainly.com/question/26416088
#SPJ4
Related Questions
Which statement best explains a difference between the interaction of light with clear glass and the interaction of light
with silver metal?
Light slows down when it interacts with glass but not when it interacts with metal.
Light slows down when it interacts with metal but not when it interacts with glass.
Most of the light passes through glass but none of the light passes through metal.
Most of the light is absorbed by glass but none of the light is absorbed by metal.
Answers
The statement that best explains a difference between the interaction of light with clear glass and the interaction of light with silver metal is C.Most of the light passes through glass but none of the light passes through metal.
What is ray of light?The ray of light caqn be described as the light that is been seen traveling in in a direction , this light could travel in a place but it it is usually through a medium.
It should be noted that when this lights become a group of light they can be regarded as the beam of light.
Learn more about light at:
https://brainly.com/question/10728818
#SPJ1
Answer:
Most of the light passes through glass but none of the light passes through metal
Explanation:
(math involved!!) In the following reaction, how many molecules of HCl are needed to produce four molecules of H2O?
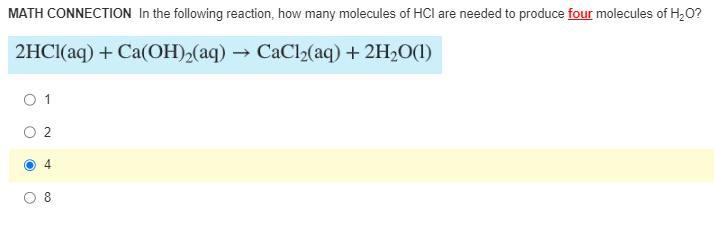
Answers
Four molecules of HCl are needed to produce four molecules of H₂O.
What is the number of molecules of of HCl needed to produce four molecules of H₂O?The number of molecules of of HCl needed to produce four molecules of H₂O is determined from the mole ratio of the reaction as given in the equation of the reaction below:
2 HCl (aq) + Ca(OH)₂ (aq) → CaCl₂ (aq) + 2 H₂O (l)
The mole ratio of the reaction as given in the equation shows that 2 moles of HCl produces 2 moles of water.
Therefore, the mole ratio of HCl and H₂O is 1 : 1
Four moles of H₂O will be produced by four molecules of HCl
The number of molecules of HCl in 1 mole of HCl is 6.02 * 10²³ molecules.
1 mole of every substance contains equal number of particles.
Hence, 1 mole of water will contain 6.02 * 10²³ molecules.
Based on the mole ratio of the reaction, four molecules of HCl are needed to produce four molecules of H₂O.
Learn more about molecules at: https://brainly.com/question/475709
#SPJ1
how do you do this
A regulation NBA basketball has a radius of 12 cm when inflated properly. What is the volume of an inflated basketball in cm^3?
someone help lol
Answers
Answer:
The volume of basketball is 7216.5 cm³.
Explanation:
Given data:
Radius of ball = 12 cm
Volume of ball = ?
Solution:
We will apply the volume formula of sphere.
V = 4/3 ×π×r³
we know that,
π = 22/7
r = 12 cm
Now we will put the values in formula.
V = 4/3 × 3.14 × (12 cm)³
V = 4/3 × 3.14 ×1728 cm³
V = 7216.5 cm³
Thus, the volume of basketball is 7216.5 cm³.
How many moles are equal to 2.4 x 1023 formula units of sodium chloride?
Answers
Answer:
The answer is 0.4 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{2.4 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{2.4}{6.02} \\ = 0.398671096...\)
We have the final answer as
0.4 molesHope this helps you
A 2.400-g sample of meat is subjected to Kjeldahl analysis. The liberated NH3(g) is absorbed by adding 50.00 mL of H2SO4(aq), which is more than enough. This occurs through the reaction 2NH3(g)+H2SO4(aq)→(NH4)2SO4(aq) The excess acid requires 19.90 mL of 0.5510 M NaOH for its complete neutralization.
Answers
Answer:
51.04% of protein in the sample
Explanation:
The question is the percentage of protein in the sample and the acid is 0.2496 M H2SO4.
First we need to calculate the moles of sulfuric acid that react:
Moles of sulfuric acid added:
0.0500L * 0.2496mol/L = 0.01248 moles of sulfuric acid.
Moles in excess:
0.01990L * 0.5510mol/L = 0.01096 moles NaOH * (1 mol H2SO4 / 2 moles NaOH) = 0.00548 moles of sulfuric acid.
Moles that react:
0.01248 moles - 0.00548 moles = 7.0x10⁻³ moles of H2SO4 react.
Based on the reaction, the moles of NH3 = Moles of N are:
7.0x10⁻³ moles of H2SO4 * (2moles NH3 / 1 mole H2SO4) = 0.014 moles NH3 = Moles N
The mass of nitrogen (Molar mass: 14g/mol):
0.014 moles N * 14g/mol = 0.196g of N.
The percentage of N is:
0.196g N / 2.400g = 8.17% of N
6.25 times the percentage of nitrogen is the percentage of protein:
8.17%*6.25 =
51.04% of protein in the sampleWhat is the major source of error in a freezing point depression experiment
a) Inaccuracy of the volume measurement b) Inaccuracy of using the balance c) Inaccuracy of thermometer d) All of the above
Answers
The major source of error in a freezing point depression experiment is inaccuracy of the thermometer used to measure the temperature change.
This is because even a small deviation in temperature measurement can significantly affect the calculated freezing point depression.
In freezing point depression experiments, a solution is prepared by dissolving a solute in a solvent, which results in a lowering of the freezing point of the solvent. The difference between the freezing point of the pure solvent and the solution is measured to determine the molar mass of the solute. However, inaccurate temperature measurement can lead to significant errors in the calculated freezing point depression. Therefore, the thermometer used should be accurate and calibrated regularly to minimize the potential for errors.
In conclusion, the inaccuracy of the thermometer used is the major source of error in a freezing point depression experiment.
To know more about freezing point depression visit:
brainly.com/question/31357864
#SPJ11
A supersaturated solution can be made to precipitate out by: A) Agitating the solution B) Adding more solute C) Both (A) and (B) D) None of the abov
Answers
A supersaturated solution can be made to precipitate out by either agitating the solution or adding more solute. Therefore, the correct answer is option C) Both (A) and (B).
A supersaturated solution is a solution that contains more solute than it can dissolve under the given conditions. As a result, it is an unstable solution that can precipitate out by various means, including agitation and adding more solute. Let's look at how this process works in more detail.
Agitating the Solution
Agitation, also known as stirring or shaking, can trigger the precipitation of a supersaturated solution. When a supersaturated solution is agitated, the solute particles suspended in the solution collide with each other and begin to clump together. These clusters of particles gradually grow larger and heavier, causing them to fall out of the solution and form a precipitate.
Adding More Solute
Another way to precipitate a supersaturated solution is to add more solute to the solution. When more solute is added, the supersaturated solution becomes even more supersaturated, reaching a state of higher instability. As a result, the solution is more likely to drop out of the solution and form a precipitate.
Both Agitating the Solution and Adding More Solute
When a supersaturated solution is both agitated and has more solute added to it, the precipitation process is accelerated. Agitation can cause solute particles to clump together, and adding more solute creates more particles that can join in the clustering process. These two processes combined can cause the supersaturated solution to reach a critical point, at which it can no longer hold all the solute in the solution and must precipitate out.
In conclusion, a supersaturated solution can be precipitated out by both agitating the solution and adding more solute. Agitation causes solute particles to collide and clump together, while adding more solute increases the instability of the solution. When these two factors are combined, they can accelerate the precipitation process and cause the supersaturated solution to fall out of the solution.The correct answer is option c.
Know more about supersaturated solution here:
https://brainly.com/question/4155452
#SPJ11
Which of the following occurs in cells only produce male and female cells

Answers
Answer:
I think its B
Explanation:
Help! What role does lactase play in breaking apart the disaccharide lactose?
lactase provides a binding site for lactose to initiate chemical breakdown.
lactase lowers the activation energy needed to begin breaking down lactose.
lactase releases heat during the breakdown of lactose.
lactase prevents too many disaccharide molecules from clumping together during chemical reactions
Answers
Lactase lowers the activation energy needed to begin breaking down lactose and is the role it plays in the reaction which is therefore denoted as option B.
What is an Enzyme?This is referred to as a biological catalyst which increases the rate of a chemical reaction and is usually specific in nature. Examples include lactase,amylase which have substrates such as lactose and starch respectively.
Lactase which is an enzyme is involved in the breaking apart of the disaccharide known as lactose by lowering the activation energy thereby increasing the rate of the reaction and ensuring that is easily done.
Read more about Enzyme here https://brainly.com/question/14577353
#SPJ1
what mass of magnesium bromide is formed when one grams of magnesium reacts with 5 g of bromine
Answers
Taking into account the reaction stoichiometry, a mass of 7.57 grams of MgBr₂ is formed when one grams of magnesium reacts with 5 g of bromine.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + Br₂ → MgBr₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleBr₂: 1 moleMgBr₂: 1 moleThe molar mass of the compounds is:
Mg: 24.31 g/moleBr₂: 159.8 g/moleMgBr₂: 184.11 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Mg: 1 mole ×24.31 g/mole= 24.31 gramsBr₂: 1 mole ×159.8 g/mole= 159.8 gramsMgBr₂: 1 mole ×184.11 g/mole= 184.11 gramsLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 24.31 grams of Mg reacts with 159.8 grams of Br₂, 1 grams of Mg reacts with how much mass of Br₂?
mass of Br₂= (1 grams of Mg× 159.8 grams of Br₂) ÷24.31 grams of Mg
mass of Br₂= 6.57 grams
But 6.57 grams of Br₂ are not available, 5 grams are available. Since you have less mass than you need to react with 1 grams of Mg, Br₂ will be the limiting reagent.
Mass of MgBr₂ formedConsidering the limiting reagent, the following rule of three can be applied: if by reaction stoichiometry 24.31 grams of Mg form 184.11 grams of MgBr₂, 1 grams of Mg form how much mass of MgBr₂?
mass of MgBr₂= (1 grams of Mg× 184.11 grams of MgBr₂)÷ 24.31 grams of Mg
mass of MgBr₂= 7.57 grams
Then, a mass of 7.57 grams of MgBr₂ can be produced.
Lear more about reaction stoichiometry:
brainly.com/question/12642627
#SPJ1
A child drops a ball from a bridge. The ball strikes the water under the bridge 2.0s later. What is the velocity of the ball when it hits the water
Answers
Answer:
20m/s
Explanation:
Given that :
Time, t = 2 seconds
Using the relation :
v = u + at
a = g is positive (downward)
V = final velocity
u = initial velocity = 0
v = u + gt
v = 0 + 10(2)
v = 20 m/s
Which of the following statements is correct? Select one: a. In adults the mature T-cell repertoire is self-renewing and long-lived and does not require a thymus for the provision of new T cells. b. T cells and B cells are both short-lived cells and require continual replenishment from primary lymphoid organs. c. The human thymus is not fully functional until age 30, at which time it begins to shrink and atrophy. d. In DiGeorge syndrome the bone marrow takes over the function of the thymus and produces mature peripheral T cells. e. None of these statements are correct.
Answers
In adults the mature T-cell repertoire is self-renewing and does not require a thymus for provision of new T cells.
Option A is correct .
When a T cell matures in the thymus, what happens to it?T-cell numbers are maintained by division of mature T cells outside of the central lymphoid organs in mature individuals, who experience a slower rate of development of new T cells in the thymus. On the other hand, even in adults, the bone marrow continues to produce new B cells.
Where do adult T cells mature?The thymus, a tiny gland in the neck, is reached by T cells as they migrate from the bone marrow. Here, they mature and separate into various sorts of Immune system microorganisms, like CD₈⁺ Lymphocytes and CD₄⁺ White blood cells.
What is the capability of mature White blood cells?T cells are a diverse and crucial class of lymphocytes that mature in the thymus and go through both positive and negative selection processes. These cells are crucial to both cell-mediated and, to a lesser extent, humoral immunity, which are components of active immunity.
Learn more about cells :
brainly.com/question/26122239
#SPJ4
2 NaOH + H2SO4 ------> 2 H2O + Na2SO4
How many moles of H2O can be produced from 12.5 moles of NaOH?
Answers
Explanation:
2moles of NaOH -> 2moles of H2O
so the number of moles r equal.
hence moles of H2O is 12.5
How many grams in 5.8 moles NaCI? with work please
Answers
\(n=\dfrac{m}{M}\) where n is moles, m is mass and M is molar mass.
To solve for mass, isolate m:
\(m=nM\)
Input given information:
\(m=5.8*58.44\\m=338.952\\m=340\)
There are 340 g in 5.8 mol of NaCl.
how many atoms are equal to 1.5 moles of hellium
Answers
Answer:
There are 1.8×1024 atoms in 1.5 mol HCl
Explanation:
Can someone talk me about molecular mass and moles?
Answers
For example NaCl,
the molar mass will be The mass of Na + the mass of Cl . The mass of NA is 22.98, and the mass of CL is 35.45 so when you add them the molar mass will equal 58.43.
That is molar mass .
Another example H20
In this case, if theres a subscript in the formula include it . This means the mass of H (hydrogen) is 1.00784, you will times that by 2 because the subscript is 2
So the MASS of H2 is 2.01568. Then you add it to the mass of oxygen which is 15.999 . Because the oxygen doesnt have a subscript you dont multiply the mass by anything . So the molar mass is 18.01.
Now moles , is just the coefficient basically ,
so in a chemical equation for example you have 2Na + Cl = 2NaCl (sodium chloride)
The moles of Sodium alone will be 2 due to the coefficient , and the moles of sodium chloride will also be 2 as they happen to have the same coefficient
Another example
2 Fe + 3 Cl2 = 2 FeCl3
The moles of Fe (iron) is 2 , the moles of Cl (chlorine) is 3 and the mole of FeCl3 is 2
Basically if they ask for the mole of an element or compound just look at the coefficient
Describe Emotions in the room after “The Vote!” during July 2, 1776 U.S History
Answers
Answer:
On July 4, 1776—the day the Declaration of Independence was approved by the Second Continental Congress—the prevailing mood was trepidation and fear.
Explanation:
How would you compare dissolving and dispersing waste materials?Dissolving them means flushing them with water; dispersing them means breaking them downDissolving them means breaking them down; dispersing them means recycling themDissolving them means breaking them down; dispersing them means spreading them outDispersing them means cleansing them; dissolving them means flushing them with water
Answers
Dissolving and dispersing are two different approaches to waste disposal. Dissolving is the method of separating and breaking down solid waste materials into smaller particles by using a solvent such as water.
Dispersing involves distributing and breaking up the waste into smaller pieces in order to make it more manageable to dispose of.
Dissolving means breaking them down; dispersing means spreading them out is the right choice.In conclusion, dissolving and dispersing waste materials have different methods. Dissolving refers to the separation and breaking down of solid waste materials into smaller particles by utilizing a solvent such as water.
Dispersing, on the other hand, involves distributing and breaking up waste into smaller pieces to make it more manageable to dispose of.
To know more about waste disposal visit:
https://brainly.com/question/30944259
#SPJ11
What is the law of conservation of mass
Answers
Answer:
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. ... If we account for all reactants and products in a chemical reaction, the total mass will be the same at any point in time in any closed system.
Answer:
the total mass of the products in a chemical reaction will always be the same as the total mass of the reactants.
Explanation:
hope this helps :)
Calculate the percent error. A block has a true mass of 16.9 g. A student masses it to be 10.5 g. What is the student's percent error?
61.0%
-61.0 %
37.9%
-37.9%
Answers
Answer:
37.9%
Explanation:
x/100 × 16.9 = 10.5
16.9x/100=10.5
16.9x=1050. (÷ b.s by 16.9)
x=62.1%
100%-62.1%=37.9%
the pressure exerted by a gas is measured to be 0.985 atm. convert this pressure to mmHg and kPa
Answers
Answer:
To convert the pressure from atm to mmHg, we can use the conversion factor:
1 atm = 760 mmHg
So, to convert 0.985 atm to mmHg:
0.985 atm x 760 mmHg/atm = 747.4 mmHg
Therefore, the pressure is 747.4 mmHg.
To convert the pressure from atm to kPa, we can use the conversion factor:
1 atm = 101.325 kPa
So, to convert 0.985 atm to kPa:
0.985 atm x 101.325 kPa/atm = 99.857 kPa
Therefore, the pressure is 99.857 kPa.
The pressure 0.985 atm is given by 99.805 kPa and 748.6 mmHg.
The three different units in chemistry are Atmospheres (atm), Millimeters of Mercury (mmHg), Pascals (Pa), or KiloPascals (kPa).
The definition of the standard atmosphere (atm), a unit of pressure, is 101325 Pa. A millimeter of mercury is a manometric unit of pressure that is currently defined as exactly 133.322 pascals. In the International System of Units (SI), the unit of pressure or stress is the Pascal (Pa).
One atm equals 101.325 kPa. To convert 0.985 atm to kPa, multiply by 101.325 kPa to get,
0.985 atm × 101.325 kPa = 99.805 kPa
One atm equals 760 mmHg. To convert 0.985 atm to mmHg, multiply by 760 mmHg to get,
0.985 atm × 760 mmHg = 748.6 mmHg
Therefore, the pressure 0.985 atm is given by 99.805 kPa and 748.6 mmHg.
Read more about Pascal:
https://brainly.com/question/30401722
pls answer this asap thankyou
Balance the chemical equation using linear algebra technique. PC15 + H₂O → H3PO4 + HCl 1) Create a matrix or put in vector form by the assignment of the variables P CI given: 1 PCl5 + 2 H₂O3 H3PO4 + 24 HCl i.e. â = H O 2) Set the equation zero and show your augmented matrix form. Ac = Ô 3) Solve the system using row operations and simplify the solutions (assign a convenient value if a free variable exists to express solutions as set of integers). 4) Enter the coefficients into the chemical equation: PC15 + H₂O H3PO4 + HCI
Answers
To balance the chemical equation PC15 + H2O → H3PO4 + HCl using linear algebra technique, we can set up an augmented matrix and solve the system of equations. Here's the step-by-step process:
Create a matrix or put it in vector form by assigning variables to P, Cl, H, O: Let's assign:
P = x (coefficient for PCl5)
Cl = y (coefficient for HCl)
H = z (coefficient for H3PO4)
O = w (coefficient for H2O)
The equation becomes: xPCl5 + yH2O → zH3PO4 + wHCl Set up the equation in matrix form:
| PCl5 | + | H2O | = | H3PO4 | + | HCl |
| 1 | + | 0 | = | 0 | + | 0 |
| 0 | + | 2 | = | 0 | + | 0 |
| 0 | + | 0 | = | 1 | + | -1 |
| 0 | + | 0 | = | 0 | + | 24 |
This gives us the augmented matrix form:
| 1 0 0 0 0 |
| 0 1 2 0 0 |
| 0 0 0 1 -1 |
| 0 0 0 0 24 |
Solve the system using row operations: Performing row operations to simplify the augmented matrix:
R2 = R2 - 2R1
R4 = R4 / 24
The simplified augmented matrix becomes:
| 1 0 0 0 0 |
| 0 1 0 0 0 |
| 0 0 0 1 -1 |
| 0 0 0 0 1 |
From the simplified matrix, we can assign values to the variables:
P = 0
Cl = 0
H = 0
O = 0
This implies that the coefficients for PCl5, H2O, H3PO4, and HCl are all zero. Enter the coefficients into the chemical equation: The balanced chemical equation is:
0PCl5 + 0H2O → 0H3PO4 + 0HCl
Therefore, the balanced equation is: PCl5 + H2O → H3PO4 + HCl (unbalanced)
To learn more about chemical, https://brainly.com/question/28792948
#SPJ11
Scientists group similar organisms together through a process called …..
Identification
Identification
Organization
Organization
Estimation
Estimation
Classification
Answers
Answer:
i think it's Organization
Explanation:
The reason being is because they obviously said 'humans' so that's why i think it is Organization
Scientists group similar organisms together through the process called classification. Each similar groups is classified into different organizations.
What is biological classification?The arrangement of species into groups according to significant similarities is known as biological classification. Then, these groups are broken down into more focused, smaller subgroups. Additional details on the species that make up each subgroup are provided.
Organisms are categorized by scientists to make them simpler to research and to facilitate information sharing. Before biologists properly comprehended genetics, the study of biological classification was conducted.
At first, scientists simply categorized creatures based on their outward appearance. Later, as genetic research developed, classification of organisms was based on the genetic and evolutionary links between them.
Therefore, scientists classify each organisms to suitable organizations.
To find more on biological classification, refer here:
https://brainly.com/question/11136571
#SPJ6
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11
what is a reaction that uses a catalyst?
Answers
One of the most commonly known catalysed reaction is that of Hydrogen Peroxide turning into Water
In this reaction, we can use Potassium Permanganate (KMnO₄) or Palladium (Pd) as a catalyst.
These catalysts helps Hydrogen Peroxide turn into water and oxygen
Catalyst: Substances that change the rate of a chemical reaction and themselves remain chemically unchanged after the reaction are called catalysts.
Example:- In the process of manufacturing of ammonia Fe is used as Catalyst.
\(N _{2} (g)+ 3H _{2} (g) \huge \rightarrow {}^{Fe}\rightarrow \small{3NH _{3}(g)}\)
Note:- there is only one arrow. I was unable to put iron over the arrow ..
Hope This Helps You ❤️1. How many liters of a 0.50 M solution are needed to give 3.5 moles of solute?
Answers
Answer:
The volume of solution in liters required to make a 0.250 M solution from 3.52 moles of solute is 14.08 liters of solution
Explanation:
The question relates to the definition of the concentration of a solution which is the number of moles per liter (1 liter = 1 dm³) of solution
Therefore we have;
The concentration of the intended solution = 0.250 M
Therefore, the number of moles per liter of the required resolution = 0.250 moles
Therefore, the concentration of the required solution = 0.250 moles/liter
The volume in liters of the required solution that will have 3.52 moles of the solute is given as follows;
The required volume of solution = The number of moles of the solute/(The concentration of the solution)
∴ The required volume of solution = 3.52 moles/(0.250 moles/liter) = 14.08 liters
The required volume of solution to make a 0.250 M solution from 3.52 moles of solute = 14.08 liters.
Therefore the number of liters required to make a 0.250 M solution from 3.52 moles of solute = 14.08 liters.
when a 24.2 ml sample of a 0.349 m aqueous acetic acid solution is titrated with a 0.308 m aqueous barium hydroxide solution, what is the ph at the midpoint in the titration?
Answers
A 24.2 mL sample of a 0.349 M aqueous acetic acid solution and a 0.308 M aqueous barium hydroxide solution were titrated, and the pH at the halfway mark was 4.76.
The midpoint of a titration occurs when the moles of acid are equal to the moles of base. At this point, the solution contains equal amounts of the conjugate acid-base pair, which acts as a buffer solution. For the titration of acetic acid with barium hydroxide, the balanced equation is:
CH₃COOH + Ba(OH)₂ → Ba(CH₃COO)₂ + 2H₂O
To find the moles of acid in the initial solution, we multiply the volume by the concentration:
moles of CH₃COOH = 24.2 mL x 0.349 mol/L = 8.43 x 10⁻³ mol
At the midpoint, we know that half of the moles of acid have reacted, leaving the other half in the form of the conjugate base, acetate ion (CH3COO⁻). Therefore, the concentration of the acetate ion is:
[CH3COO⁻] = 8.43 x 10⁻³ mol / 2 / 24.2 mL = 0.174 M
To find the pH at the midpoint, we can use the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
The pKa of acetic acid is 4.76, and at the midpoint, the concentration of the conjugate acid (acetic acid) is equal to the concentration of the conjugate base (acetate ion). Therefore,
pH = 4.76 + log([0.174 M]/[0.174 M]) = 4.76
Thus, the pH at the midpoint of the titration of a 24.2 mL sample of a 0.349 M aqueous acetic acid solution with a 0.308 M aqueous barium hydroxide solution is 4.76.
To learn more about acetic acid refer to:
brainly.com/question/15202177
#SPJ4
2500m into kilometer
Answers
1 meter = 1000 km
2500 meter = 2500/1000 km
= 2.5 km
Imagine that you traveled to the Moon for a vacation and discovered that your weight is different there. How would your weight be different on the Moon than on Earth? Why would it be different? Does this mean that you ""lost weight"" and need to buy new clothes that fit? Why or why not?
Answers
Answer
No you did not lose weight.
Explanation:
Weight is the force acting on an object, Gravity is a force on earth which does not apply to the moon so your weight may change depending in where you are but your mass will stay the same because mass measures the matter of an object ITSELF! Have a nice day....
Answer:
I would take my weight on Earth and divide it by 6. I'd weigh less because the gravitational pull on the Moon is less, and, weight is the measure of gravitational pull. I wouldn't "loose weight" because I'd weigh the same on Earth as I did the day I left (give or take a few pounds).
Explanation:
What’s different about ionic bonding and covalent bonding ? (Help I need the answer ASAP)