Calculate each of the following quantities:
(a) Mass (g) of 0.346 mol of Zn
Answers
The mass of the 0.346 mol of Zn atoms is 22.62148 moles.
What is mass?Mass is a physical body's total amount of matter. It is computed by multiplying the molar mass by the number of moles. The SI unit is kg and grams, and it is symbolized by the letter m.
Zn is a chemical element that is required for the body at a minimum amount
Given that number of moles is 0.346 mol of Zn
The molar mass of the Zn is 65.38 u
By the formula of mass
mass = molar mass x moles
Putting the value in the formula:
0.346 mol x 65.38 = 22.62148 moles
Thus, the mass of the 0.346 mol of Zn atoms is 22.62148 moles.
To learn more about mass, refer to the link:
brainly.com/question/4220551
#SPJ4
Related Questions
Balance the chemical equation : CH₄ + O₂ → CO₂ + H₂O
Answers
Answer:
balanced equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Explanation:
Given sample: CH₄ + O₂ → CO₂ + H₂O
The carbon is same, so no need to change. Hydrogen 2 less on left side so putted "2" before H₂O = "2H₂O"so now, there is total 4 oxygen on left side to balance put 2 before right side oxygen like this "2O₂"changes applied: CH₄ + 2O₂ → CO₂ + 2H₂O
¿Qué podría pasar si la fuerza fuerte no existiera?
Answers
Answer:
andariamos flotando
Explanation:
Which of the following is NOT a function of the skeletal system?
A. Exchanges oxygen and carbon dioxide
B. Produce red blood cells
C. Protects the organs
D. Stores calcium and phosphorus
(This is a science question btw)
Answers
Answer:
D. Stores calcium and phosphorus
Explanation:
Correct me if I'm wrong
which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f7
Answers
Answer:
Lawrencium (Lr)
Explanation:
The element with the given electron configuration is Lawrencium (Lr), which has an atomic number of 103.
Benzene used to be a common solvent in organic chemistry, but is no longer used because it is a suspected carcinogen. Benzene undergoes metabolic oxidation by cytochrome P450 enzymes to form an electrophilic epoxide which can alkylate proteins and DNA. Toluene is also oxidized by cytochrome P450 enzymes, but the metabolite is less toxic and is rapidly excreted. Suggest what the metabolite might be and why the metabolism of toluene is different from that of benzene?
Answers
The metabolite of toluene that is formed via oxidation by cytochrome P450 enzymes is benzyl alcohol. This metabolite is less toxic than the electrophilic epoxide formed from benzene oxidation, and is rapidly excreted from the body.
The reason for the difference in metabolism between toluene and benzene lies in their respective chemical structures. Benzene has a planar, cyclic structure with delocalized pi-electrons, making it highly reactive and capable of forming electrophilic species that can damage biological molecules.
Toluene, on the other hand, has a methyl group attached to the benzene ring, which makes it less reactive than benzene. This methyl group serves to protect the aromatic ring from oxidation, resulting in the formation of a less toxic metabolite in the case of toluene oxidation.
Additionally, the metabolism of toluene can be further modified by conjugation with glucuronic acid, which enhances its rapid excretion from the body.
learn more about electrophilic here:
https://brainly.com/question/31182532
#SPJ11
Plants in forests take up carbon dioxide from the atmosphere during photosynthesis. They transform the carbon dioxide into plant material. When plants die, their organic matter is often worked into the soil by decomposers. Some of this organic matter remains within the soil and forest floor, and some of it is taken up by other living things.
Based on this information, what role do forests play in the carbon cycle?
A.
Forests are carbon sinks because they store carbon.
B.
Forests are carbon sinks because they do not absorb carbon dioxide when plants die.
C.
Forests are carbon sources because they emit carbon.
D.
Forests are carbon sources because they can be burned to emit carbon dioxide.
Answers
Because Trees store carbon and make it oxygen
Answer: B. forests are carbon sinks because they store carbon
Explanation:forests take up carbon from the atmosphere through photosynthesis and from organic matter through decomposition. so, forests are carbon sinks because they store carbon
What causes the liquid in a thermometer to rise?
Answers
Answer:
The level of colored liquid rises when the thermometer is placed in hot water. Heat causes the molecules of the liquid to get farther apart. The molecules of the liquid break down into atoms and take up more space.
Explanation:
Convert 595 grams of KBr to moles of KBr. One mole of KBr weighs 119 grams.
Convert: 595 g KBr
To: mol KBr
Conversion factor: 1 mol KBr = 119 g KBr
Round your answer to the nearest while and enter the number only in the box below (no units)

Answers
Answer:
595 g will produce 5 mol of KBr
Explanation:
1 mol KBr = 119 gB mol KBr = 595 gcross multiply119B = 595B = 595/119B = 5 molMole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 5 moles are there in 595 grams KBr.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically, the formula for number of mole can be given as
number of mole of KBr =given mass of KBr ÷ molar mass of KBr
Molar mass of 1 mole of KBr= 119 g/mol
mass of KBr= 595 grams
substituting all the given values in the above equation, we get
number of mole of KBr= 595 ÷ 119
On calculation, we get
number of mole of KBr = 5 moles
Therefore, 5 moles are there in 595 grams KBr.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ5
Hi!, please help :)
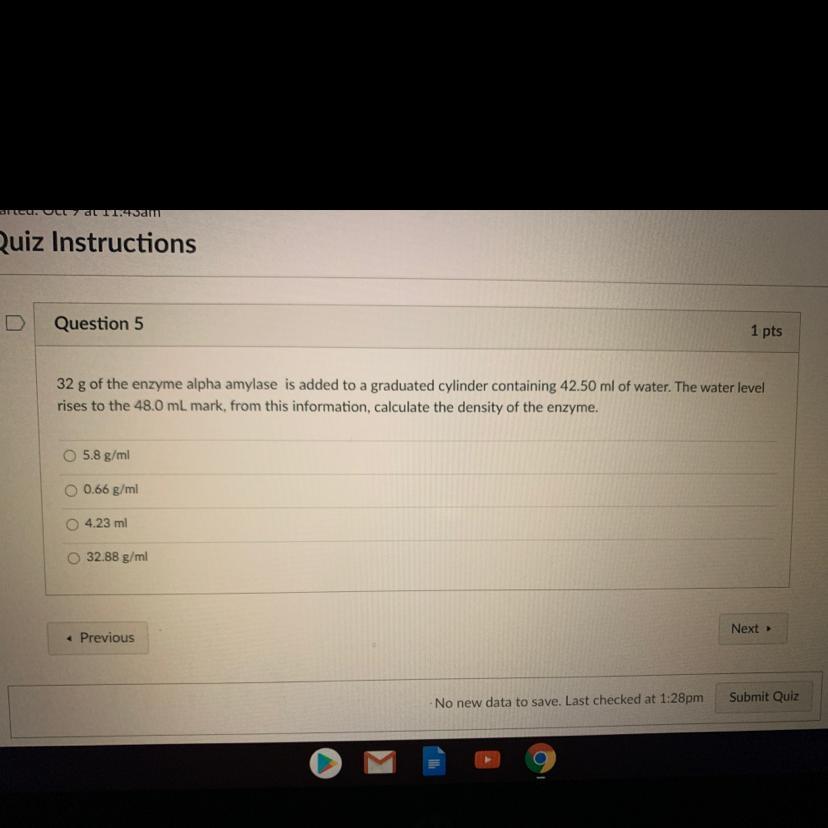
Answers
Answer:
5.8
Explanation:
what state of matter has more volume?
Answers
Answer:
please mark as brainliest
Explanation:
ans: Gas
The particles of matter in the gaseous state are neither close together nor fixed in place. The gas expands to fill its container. Thus, a given number of particles will have the greatest volume in the gas state.30/06/2014
what would happen to dietary protein if hcl were not being produced in sufficient quantities?
Answers
Dietary protein will not be digested if Hcl were not being produced in sufficient quantities.
In the stomach, there are some glands found on the stomach lining that helps to release hydrochloric acid (HCl). Their major role is to dissolve food and produce an acidic medium.
The acidic media promotes and improves the conversion of pepsinogen to pepsin. Pepsin acts as an enzyme that supports protein digestion.
Protein digestion requires a pH of around 1.8 which can be achieved by the secretion of HCl.
As a result, if HCI is not released in the stomach in sufficient quantities, pepsin will not be activated and it would have a detrimental impact on protein digestion.
Therefore, we can conclude that dietary protein will not be digested if Hcl were not being produced in sufficient quantities.
Learn more about dietary proteins here:
https://brainly.com/question/17303328?referrer=searchResults
If liquids cannot burn, what makes them flammable? Give an example of a flammable
liquid.
Answers
Explanation:
its the vaporizer that air brun
Hypothesis: How easily for you think the following substances are fermented by yeast?
Answers
Yeast is a type of fungus that can ferment certain substances, meaning it breaks down sugars and converts them into alcohol and carbon dioxide. The ease with which a substance is fermented by yeast depends on a few factors, including the type of yeast being used and the composition of the substance itself.
Generally speaking, substances that contain a high amount of simple sugars are more easily fermented by yeast. This is because yeast is able to quickly and efficiently break down these sugars into alcohol and carbon dioxide. Examples of substances that are easily fermented by yeast include fruit juices, honey, and molasses.
On the other hand, substances that are more complex or contain less sugar may be more difficult for yeast to ferment. For example, yeast may have a harder time breaking down starches, such as those found in grains, without additional processing steps.
It's worth noting that different strains of yeast may also have varying levels of ability to ferment certain substances. Some strains may be better suited for fermenting certain types of beer or wine, for example, while others may be more effective at fermenting bread dough.
Overall, the ease with which a substance is fermented by yeast depends on a variety of factors, and may require some trial and error to determine the best approach for a particular substance.
Learn more about simple sugars here:
brainly.com/question/984360
#SPJ11
Students performing a similar analysis to your lab made several mistakes in their experiments. Explain how each error affected the calculated Molarity of the NaOH solution. Hint: start with deciding how the error will influence the quantities used in the equation for Molarity (Molarity = moles / Liter). Student A did not record the correct amount of KHP to use in the titration. The scale reading was 0.15 grams of KHP, but the student recorded the value as 0.10 grams of KHP. The student used the quantity 0.10 grams for the calculation.
Answers
Answer:
See explanation
Explanation:
The reaction of NaOH and KHP is a neutralization reaction. The molarity of KHP is often calculated from the mass of KHP used to prepare the standard solution.
KHP is a slightly acidic substance. It is used as a primary standard for acid-base titrations because the solid is stable in air hence it is easy to weigh accurately. Also, the solid is neither hygroscopic nor deliquescent.
If student A recorded the amount of KHP used for the titration as 0.10g instead of 0.15g, then the molarity of NaOH calculated will be less than the actual the actual molarity of the NaOH.
Choose the INCORRECT statement/statements?
(i) The brain stem joins the brain with the spinal cord.
(ii) The right hemisphere of the brain controls the left side of the body.
(iii) Our sense organs are controlled by cerebellum.
(iv) The pigments in our pupil give colour to the eyes.
Only (i) and (ii)
Only (ii) and (iii)
Only (i) and (iii)
Only (iii) and (iv)
Answers
Answer:
iv it does not make but if im wrong dont report please!
the carbon cycle model is shown here. certain processes in this process are considered 'fast' cycling of carbon. which components of the carbon cycle would be classified as fast processes? select all that apply.
Answers
The components of the carbon cycle that would be classified as fast processes include:1. Photosynthesis 2. Respiration
3. Decomposition of organic matter 4. Dissolution of CO2 in the ocean 5. Release of CO2 by burning fossil fuels.
The carbon cycle is the process by which carbon, a crucial element for life on Earth, is exchanged and recycled among different reservoirs, such as the atmosphere, oceans, land, and living organisms. Some processes in the carbon cycle are considered fast processes due to their relatively rapid rates of carbon exchange.
Photosynthesis: Photosynthesis is the process by which plants and other photosynthetic organisms use sunlight, carbon dioxide (CO2), and water to produce organic matter and release oxygen.
Respiration: Respiration is the process by which living organisms, including plants, animals, and microorganisms, release energy by oxidizing organic carbon compounds and producing CO2 as a byproduct.
Decomposition of organic matter: Decomposition is the breakdown of dead organic matter by microorganisms, such as bacteria and fungi, into simpler organic compounds and eventually into CO2.
Dissolution of CO2 in the ocean: Dissolution of CO2 in the ocean is a fast process in which atmospheric CO2 dissolves into the surface waters of the ocean, forming dissolved carbon species such as bicarbonate and carbonate ions.
Release of CO2 by burning of fossil fuels: Burning of fossil fuels, such as coal, oil, and natural gas, releases CO2 into the atmosphere as a byproduct of combustion.
Learn more about carbon cycle here:
https://brainly.com/question/30508567
#SPJ11
Briefly explain how you will isolate p tert butylphenol.
Answers
Isolation of p-tert-butylphenol: p-tert-butylphenol can be obtained from the reaction between p-cresol and isobutylene. It can be isolated by steam distillation followed by the crystallization process of the aqueous layer, which is used to precipitate the product from the solution.
The solvent is then evaporated under reduced pressure to obtain the product
The isolation of p-tert-butylphenol is a process that needs to be carried out carefully. The following steps can be used:
Steam distillation: A steam distillation process can be used to extract p-tert-butylphenol. This method is effective because p-tert-butylphenol is insoluble in water, but it will still be carried over by the steam.
The crude product is obtained by steam distillation of the reaction mixture
Crystallization: After obtaining the crude product, the aqueous layer is used to precipitate the product from the solution. The mixture is then cooled to room temperature, and crystals of p-tert-butylphenol start to form. The crystals are then filtered and washed with cold water to remove any impurities.
Solvent evaporation: After washing the crystals, the solvent is evaporated under reduced pressure. The remaining solid is then recrystallized in hot water and dried under vacuum.
Briefly, the isolation of p-tert-butylphenol involves steam distillation followed by the crystallization process of the aqueous layer, which is used to precipitate the product from the solution. The solvent is then evaporated under reduced pressure to obtain the product.
To learn more about crystallization, visit:
https://brainly.com/question/32130991
#SPJ11
PLease answer
Physical or chemical change?
Mothballs in a drawer disappear
Answers
which change in the blood chemistry causes an increase in respiration?
Answers
There are several changes in blood chemistry that can lead to an increase in respiration.
One of the most significant factors is the buildup of carbon dioxide in the bloodstream. As carbon dioxide levels rise, the body's respiratory system responds by increasing the rate and depth of breathing to expel excess CO2 and maintain proper blood pH levels.
Another factor that can increase respiration is a decrease in oxygen levels in the blood. When oxygen levels drop, the body attempts to compensate by breathing faster and deeper to take in more oxygen. This response is particularly important in situations where oxygen delivery to the body's tissues is compromised, such as during exercise or at high altitudes.
Overall, respiration is closely tied to blood chemistry, with many different factors influencing how and why we breathe. By maintaining a delicate balance of gases and nutrients in the bloodstream, our bodies are able to efficiently extract oxygen and eliminate waste products, ensuring that our cells and tissues receive the oxygen they need to function properly.
To know more about blood chemistry visit:
https://brainly.com/question/30453877
#SPJ11
In CaSO4, the oxidation number of Ca is
that of S is
and that of O is
Answers
Answer: In CaF2, the oxidation number of Ca is +2
, and that of F is -1
. In H2SO4, the oxidation number of H is +1
, that of S is +6
, and that of O is -2
. In CaSO4, the oxidation number of Ca is +2
, that of S is +6
, and that of O is -2
. In HF, the oxidation number of H is +1
, and that of F is -1
A metal crystallizes in a face centered cubic structure and has a density of 11. 9 g/cm3. If the radius of the metal atom is 138 pm, what is the identity of the metal?.
Answers
The metal is palladium. The density of a face-centered cubic (fcc) crystal can be calculated using the following equation:
ρ = (z * M) / (a^3 * N_A)
Where:
ρ is the density in g/cm^3
z is the number of atoms per unit cell
M is the molar mass of the metal in g/mol
a is the edge length of the unit cell in cm
N_A is Avogadro's number (6.022 x 10^23 atoms/mol)
We know that z = 4 for an fcc crystal, M = 106.42 g/mol for palladium, and a = 2(138 pm)/10^-12 = 1.422 Å = 1.422 x 10^-8 cm.
Plugging these values into the equation, we get:
ρ = (4 * 106.42 g/mol) / (1.422 x 10^-8 cm)^3 * 6.022 x 10^23 atoms/mol) = 11.9 g/cm^3
Therefore, the identity of the metal is palladium.
To know more about face centered cubic structure, click here:-
https://brainly.com/question/13352445
#SPJ11
The mass of an object is 24g and the volume of the object is 16 mL. Calculate the
density of the object?
Answers
Answer:
D = m/v
Explanation:
So m = 24g and v = 16mL. (I assume these are the units being used). Then, we get D = 24g/16mL which is 1.5 g/mL
how many valence electrons does tungsten have
Answers
Answer:74
Explanation:
Answer:
I believe Tungsten has 2 valence electrons.
Explanation:
Hope this helps!
g a 25.0-ml sample of 0.10 m hcl is titrated with 0.10 m naoh. what is the ph of the solution after 12.7 ml of naoh have been added to the acid? please report with 1 decimal place.
Answers
A 25.0-ml sample of 0.10 M HCl is titrated with the 0.10 M NaOH. The pH of the solution after the 12.7 ml of NaOH have been added to the acid is 1.4.
The moles of the HCl = molarity × volume
The moles of the HCl = 0.10 × 0.025
The moles of the HCl = 0.0025 mol
The moles of the NaOH = molarity × volume
The moles of the NaOH = 0.10 × 0.0127
The moles of NaOH = 0.00127 mol
HCl + NaOH ----> NaCl + H₂O
0.0025 mol of the HCl react with the 0.0025 mol
Remaining moles = 0.0025 - 0.00127
= 0.00123 mol
[H⁺] = 0.00123 / ( 0.025 + 0.0127)
= 0.033 M
pH = - log [H⁺]
pH = 1.4
To learn more about pH here
https://brainly.com/question/9408394
#SPJ4
applications of anaerobic respiration
Answers
Applications of anaerobic respiration is generating microbial fuel cell
Anaerobic respiration is the because of lack of oxygen they carry out respiration in the absence of oxygen to produce the energy they require called as anaerobic respiration
Anaerobic respiration is useful generating microbial fuel cell which employ bacteria that respire solid electron acceptor to transfer electron from reduced compound to an electrode this process can simultaneously degrade organic carbon waste and generate electricity
Know more about application
https://brainly.com/question/8185902
#SPJ1
how many moles are in 7.90g of C
Answers
The number of amount of moles contained in 7.90 grams of carbon is 0.66 moles.
How to calculate number of moles?Mole is the base unit of amount of substance i.e. the amount of substance of a system which contains exactly 6.02214076 × 10²³ elementary entities.
The number of moles in a substance can be calculated by dividing the mass of the substance by its molar mass as follows:
no of moles = mass ÷ molar mass
According to this question, 7.90g of carbon is given.
molar mass of carbon is 12g/mol
moles = 7.90g ÷ 12g/mol
moles = 0.66 moles
Learn more about moles at; https://brainly.com/question/26416088
#SPJ1
sources of chemical raw material s
Answers
Fossil fuels (coal, natural gas, and petroleum), air, water, salt, limestone, sulphur, and some specialty raw materials for special goods, like phosphates and the mineral fluorspar, are all examples of raw materials.
What is the primary raw material?Sand (including silica sand), clay, hard rock, limestone (including metallurgical limestone), gravel, and other building and road-building supplies are considered basic raw materials (BRM). It also includes elements used to improve agricultural soil, such as limesand and gypsum.
How many different kinds of raw materials occur?Direct and indirect raw materials can be separated into two categories. The finished product contains direct materials. Examples are the fabric used to produce clothing or the wood used to make furniture. Throughout the process, indirect materials are used.
To know more about raw material visit:
https://brainly.com/question/13376768
#SPJ9
The complete question is -
What are the sources of chemical raw material ?
Find the pH of each solution:
a) 0.20 M KCHO2 (Ka(HCHO2)=1.8×10−4
b) 0.23 M CH3NH3I (Kb(CH3NH2)=4.4×10−4
c) 0.19 M KI
Answers
The concentration of H⁺ ions is 1.19 x 10⁻³ M, and the pH is: 2.92, the concentration of H⁺ ions is 3.36 x 10⁻³ M, and the pH is: 2.47, the concentration of H⁺ ions is 3.1 x 10⁻⁹ M, and the pH is 8.51.
For KCHO₂, the dissociation reaction is: KCHO₂ + H₂O ↔ HCHO₂ + KOH
The equilibrium constant expression for reaction is:
Ka = [HCHO₂][OH⁻]/[KCHO₂]
Since KOH is a strong base, we can assume that all of it will react with the H⁺ ions from HCHO₂, leaving [OH⁻] = [KOH]. Thus, we can write:
Ka = [HCHO₂][KOH]/[KCHO₂]
Since [KOH] is equal to the initial concentration of KCHO₂ (0.20 M), and assuming that x is the concentration of HCHO₂ that dissociates, we can write:
Ka = x² / (0.20 - x)
Solving for x, we get:
x = sqrt(Ka*(0.20 - x)) = 1.19 x 10⁻³ M
Thus, the concentration of H⁺ ions is 1.19 x 10⁻³ M, and the pH is:
pH = -log[H⁺] = 2.92
For CH₃NH₃I, we need to first write the hydrolysis reaction:
CH₃NH₃⁺ + H₂O ↔ CH₃NH₂ + H₃O⁺
The equilibrium constant expression for reaction is;
Kb = [CH₃NH₂][H₃O⁺]/[CH₃NH₃⁺]
Since CH₃NH₃⁺ is the weak acid and CH₃NH₂ is the conjugate base, we can assume that [CH₃NH₃⁺] ≈ initial concentration of CH₃NH₃I (0.23 M). Thus, we can write:
Kb = x² / (0.23 - x)
where x is the concentration of CH₃NH₂ that forms. Since CH₃NH₂ is a weak base, we can assume that [H₃O⁺] ≈ 0. Thus, we can solve for x:
Kb = x² / (0.23 - x)
4.4 x 10⁻⁴ = x² / (0.23 - x)
x = 3.36 x 10⁻³ M
Thus, the concentration of H⁺ ions is 3.36 x 10⁻³ M, and the pH is:
pH = -log[H⁺] = 2.47
For KI, we need to consider the reaction of I- with water:
I⁻ + H₂O ↔ HI + OH⁻
The equilibrium constant expression for reaction is;
Kw/Kb = [HI][OH⁻]/[I⁻]
Since HI is a strong acid, we can assume that [HI] ≈ 0. Thus, we can write:
Kw/Kb = [OH⁻]/[I⁻]
where Kw is the ion product constant for water (1 x 10⁻¹⁴), and Kb is the base dissociation constant for I-. Solving for [OH⁻], we get:
[OH⁻] = (Kw/Kb) [I⁻] = (1 x 10⁻¹⁴)/(5.9 x 10⁻¹⁰) [0.19] = 3.22 x 10⁻⁶ M
Thus, the concentration of H⁺ ions is:
[H+] = Kw/[OH⁻] = 3.1 x 10⁻⁹ M
and the pH is:
pH = -log[H⁺] = 8.51
To know more about pH here
https://brainly.com/question/491373
#SPJ4
The earth formed 4600 million years ago. What percentage of the Earth’s age has the atmosphere been its current composition
Answers
Answer: 4.35%
Explanation:
Scientists estimate that the current composition of gases in the Earth's atmosphere has been stable for the last 200 million years or so.
If the Earth was formed 4,600 million years ago then the percentage of her age that the atmosphere has been in its composition is:
= 200 / 4,600 * 100%
= 4.35%
5. Complete and balance an equation for each reaction:
CaI₂ + Hg(NO₃)₂ ---------> (HgI₂ precipitates)
Al + Cl₂ --------->
Ag + HCl ------->
C₂H₂ + O₂ ------->
MgCl₂ --------->
Answers
CaI₂ + Hg(NO₃)₂ --------->HgI₂ + Ca(NO3)2
2Al + 3Cl₂ --------->2AlCl3
Ag + HCl ------->AgCl + H2
C2H2 + 5O2 --------> 4CO2 + 2H2O
MgCl₂ --------->Mg + Cl2