By definition, a(n) _______acid is any species that can donate a proton. Ammonia has a proton bonded to nitrogen, so ammonia can be an base (although a very weak one). A(n)___________ base is a proton acceptor, that is, it must have a pair of electrons to share with a proton. In theory, any atom with an unshared electron pair can be a________ pair so ammonia is a_______ proton; instead, it acts as a base and pulls a proton from water to a small extent. base The nitrogen in ammonia has an unshared electron In water, ammonia is too weak an ______-to give up its
Answers
Answer:
A Brønsted-Lowry acid.
A Brønsted-Lowry base.
Ammonia is an acceptor of proton.
Explanation:
A Brønsted-Lowry acid is any atom that can donate a proton (H +) to another atom or molecule whereas Brønsted-Lowry base is any species that can accept a proton from another atom or molecule or in other words, a Brønsted-Lowry acid is a proton donor, while on the other hand, a Brønsted-Lowry base is a proton acceptor. The ammonia molecule accepts the hydrogen ion is considered as the Brønsted-Lowry base.
Related Questions
I need help I don’t understand this is hitting

Answers
Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Thus, They are additionally known as limiting reactants or limiting agents. A predetermined quantity of reactants are necessary for the reaction to be completed, according to the stoichiometry of chemical reactions.
In the aforementioned reaction, 2 moles of ammonia are created when 3 moles of hydrogen gas react with 1 mole of nitrogen gas.
In most cases, this reactant dictates when the reaction will end. The reaction stoichiometry can be used to determine the precise quantity of reactant that will be required to react with another element. The limiting agent is determined by the mole ratio rather than the mass of the reactants.
Thus, Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Learn more about Chemical reaction, refer to the link:
https://brainly.com/question/22817140
#SPJ1
How many moles of water contain each of the following number of molecules?
4.38 × 10^21 molecules
Report your answer using appropriate number of significant figures.
Answers
In 4.38 × 10^21 molecules of water, there are approximately 0.073 moles.
To calculate the number of moles, we can use Avogadro's number, which states that 1 mole of a substance contains 6.022 × 10^23 molecules. So, by dividing the given number of molecules (4.38 × 10^21) by Avogadro's number, we can find the number of moles.
Now, let's explain the process in detail. Avogadro's number is a constant that represents the number of particles (atoms, molecules, etc.) in one mole of a substance. It is approximately 6.022 × 10^23. Therefore, if we divide the given number of molecules by Avogadro's number, we can determine the number of moles.
In this case, we divide 4.38 × 10^21 molecules by 6.022 × 10^23 molecules/mole, resulting in approximately 0.073 moles.
Significant figures play an important role in reporting the answer. The given number of molecules has three significant figures (4, 3, and 8), so our answer should be reported with three significant figures as well. Therefore, the number of moles is approximately 0.073.
for such more questions on molecules
https://brainly.com/question/475709
#SPJ8
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
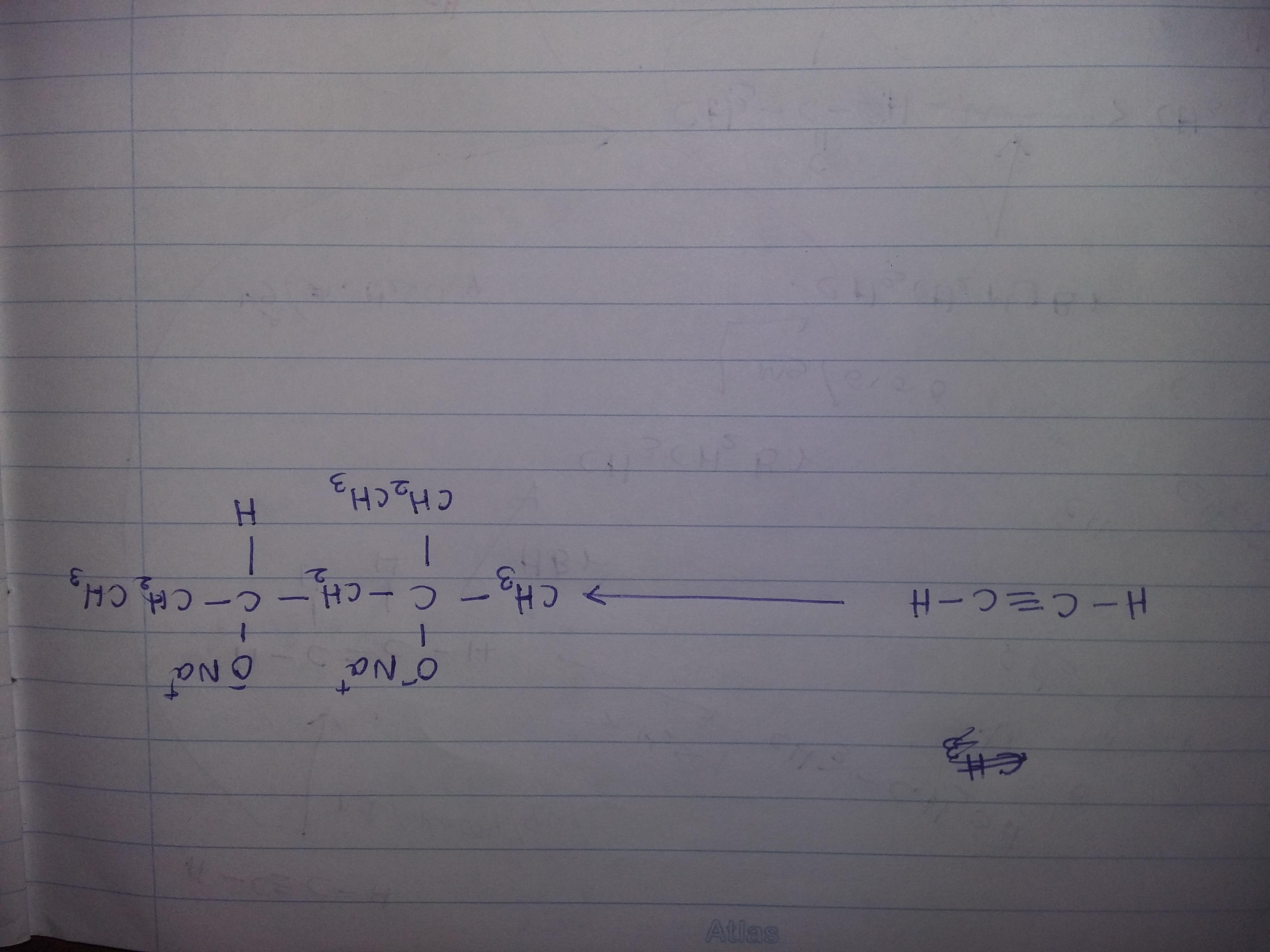
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
When an ionic compound is created, energy is _________when creating the cation, energy is usually _______ when creating the anion, and energy is _______ when the ionic bond is formed resulting in a net [ Select ] of energy for the entire process of making an ionic compound.
Answers
Answer:
Absorbed
Released
Released
Explanation:
The formation of a cation is an endothermic process because energy must be absorbed in order to remove an electron from an atom.
Similarly, energy is evolved when an electron is added to an atom to form a negative ion.
The formation of an ionic compound is an exothermic process. Since ionic compounds are more stable than the individual ions separated by a distance, the excess energy of the isolated ions is evolved when the ionic compound is formed.
which is an example of a colloid? a mixture that settles out, a mixture that scatters light, a mixture that is separated by filtration, or a salt and water mixture?
Answers
These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Option B)
A colloid is a type of mixture in which particles are dispersed throughout a medium, creating a homogeneous appearance. Unlike solutions, where the particles are completely dissolved, and suspensions, where the particles settle out, colloids have particles that are larger than those in solutions but smaller than those in suspensions. One characteristic of colloids is that they can scatter light due to the size of the particles. This scattering of light is known as the Tyndall effect. Examples of colloids include milk, fog, and aerosol sprays. These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Therefore option B) is correct
For more question on mixture
https://brainly.com/question/24647756
#SPJ8
Note Complete Question
which is an example of a colloid?
a mixture that settles out,
b mixture that scatters light,
c mixture that is separated by filtration,
d salt and water mixture?
a compound is found to be 51.38% carbon, 8.64% hydrogen, and 39.97% nitrogen. it has a molecular molar mass of 140,22 g/mol
Answers
The molecular compound in the given statement is CH₆N₂.
What defines a molecular compound?Chemical compositions of molecules that make up molecular compounds reflect the number of elements that are really bound together within the molecule. Due to the sides and angles of a atoms' links to one another, the complex has a certain structure.
Briefing:Consider 140.22 g/mol of compound
Carbon = 140.22 * 51.38/100
Carbon = 72 gm = 1 mol
Hydrogen = 140.22 * 8.64/100
Hydrogen = 12 gm = 6 mol
Nitrogen = 140.22 * 39.97/100
Nitrogen = 56 gm = 2 mol
The molecular compound is CH₆N₂.
To know more about Molecular compound visit:
https://brainly.com/question/23088724
#SPJ1
The complete question is -
A compound with molar mass 140.22g/mol is known to contain 51.38% carbon, 8.64% hydrogen and 39.97% nitrogen. Find the molecular of this compound.
What is the IUPAC name of the following substance?

Answers
The International Union of Pure and Applied Chemistry (IUPAC) provides a standard system for naming organic compounds.
It is essential to learn this nomenclature system to communicate correctly about the chemical structures of compounds and how they relate to each other. Here is the IUPAC name of the following substance.Below is the structure of the given compound: In the given compound, there are four carbon atoms that are connected with single bonds. Carbon atoms are also attached to hydrogen atoms. Since it has four carbons in the main chain, the root name will be "but-". The functional group present in the molecule is the carboxylic acid group (-COOH), which gives the suffix "-oic acid." Therefore, the IUPAC name of the given substance is Butanoic acid.Thus, the IUPAC name of the given compound is Butanoic acid. It is essential to know the IUPAC naming of organic compounds to communicate correctly about the chemical structures of the compounds.
for such more questions on Chemistry
https://brainly.com/question/29886197
#SPJ8
which substance has nonpolar covalent bonds
Answers
Nonpolar covalent bonds are formed when electrons are shared equally between two atoms.
A non-polar covalent bond is a type of chemical bond that is formed when electrons are generally shared equally between two atoms. Thus, in an atom, the number of electrons shared by the adjacent atoms will be the same.
The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible or very less. It further means that there is no separation of charges between the two atoms or both the atoms have similar electronegativity. This type of bond is also formed when atoms that share a polar bond arrange themselves in such a manner where the electric charges tend to cancel each other out.
A non-polar covalent bond can occur between two identical non-metal atoms or between different atoms.
To know more about nonpolar covalent bonds
https://brainly.com/question/28955235
#SPJ4
which phrase is the best description of osmosis
Answers
movement of water from high potential to low potential
Type the correct answer in the box.
Calculate the density of the substance.
A sample of a substance has a mass of 27.3 grams and a volume of 7.0 centimeters3. The density of this substance is ____
grams/centimeter3.
Answers
Answer:
3.9g/cm3
Explanation:
Density ( d)=?
Mass(m)=27.3g
Volume (v)=7.0cm3
D=m÷v
D=27.3g÷7.0cm3
D=3.9g/cm3
A patient order requires 1.6 L of a 0.5 % benzalkonium chloride antiseptic. You have a 13 % stock solution. How much stock solution will you need to prepare the order?
Answers
The amount of stock solution that would be needed to prepare the required solution would be 0.06 L.
DilutionThe problem can be solved using the dilution principle. According to this principle, before and after diluting a particular solution, the number of moles of solutes in the solution must remain the same.
The dilution principle is expressed as an equation as shown below:
\(m_1v_1\) = \(m_2v_2\)
Where:
\(m_1\) = molarity before dilution (in this case, 13%)
\(v_1\) = volume before dilution (what we want to determine)
\(m_2\) = molarity after dilution (in this case, 0.5%)
\(v_2\) = volume after dilution ( in this case, 1.6 L)
Using the provided information, the volume of the stock solution to be taken can be calculated as follows:
\(v_1\) = \(m_2v_2\)/\(m_1\)
= 0.5 x 1.6/13
= 0.06 L
In other words, 0.06 L of the stock solution would be required. This volume will be diluted to the 1.6 L mark with water in order to have the desired solution.
More on dilution can be found here: https://brainly.com/question/21323871
#SPJ1
1. Which of the following equilibriums are homogeneous and which are heterogeneous?
a. 2HF(g) ⇌ H2(g)+F2(g)
b. C(s) + 2H2(g) ⇌ CH4(g)
c. H2CCH2(g) + H2(g) ⇌ C2H6(g)
d. 2Hg(l) + O2(g) ⇌ 2HgO(s)
Answers
Explanation:
a. homogeneous equilibrium (all species are in the gas phase)
b. heterogeneous equilibrium (solid carbon is present)
c. heterogeneous equilibrium (solid catalyst may be present)
d. heterogeneous equilibrium (liquid mercury and solid mercury(II) oxide are present)
Drag the words to the correct locations to match the three main components of an aquaponics system with their functions.

Answers
Answer:these remove the nitrate from. The water - plants
These produce waste that contains ammonia- fish
These convert ammonia into nitrate-bacteria
Explanation:
Plants remove the nitrate from the water, fishes produce waste that contains ammonia, and bacteria convert ammonia into nitrate.
An aquaponics system is a sustainable method of agriculture that combines aquaculture with hydroponics. In this system, fish or other aquatic animals are kept in a tank or pond. The fish produce waste in the form of ammonia-rich water.
This wastewater is then circulated to the hydroponic component of the system, where plants are grown in a soil-less medium or directly in water. Beneficial bacteria in the system convert ammonia into nitrites and then nitrates, which are essential nutrients for plants.
Learn more about aquaponics systems, here:
https://brainly.com/question/13885348
#SPJ1
I need to mark the steepest areas.

Answers
The hilltops and the steepest point of a slope are found in the highest and most closely-spaced contour lines on the map.
What are contour lines?Contour lines are lines on a map that connect points of equal elevation. Each contour line represents a specific elevation, and the spacing between contour lines represents the slope of the terrain.
The closer the contour lines are to each other, the steeper the slope. The hilltops are found where the contour lines form a closed circle or oval shape. The steepest point of a slope is found where the contour lines are closest together, indicating the highest rate of change in elevation.
Learn more about contour lines at: https://brainly.com/question/13088900
#SPJ1
help answer this 50 points for each page!!!!


Answers
Thanks for the points, I need them to help other people,
Thanks,
Tqkeoi.
8.50 of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 160./gmol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 14.03g water 3.83g
Answers
The compound would have a molecular formula of C5H10O2.
What is the empirical formula of the compound?
The empirical formulas is the simplest formula of a compound.
We know that;
Mass of the carbon = 14.03 * 12/44 = 3.82 g
Moles of C = 3.82 g/12 g/mol = 0.32 moles
Mass of water = 3.83 * 2/18 = 0.43 g
Moles of H = 0.43 g/1 g/mol = 0.43 moles
Mass of oxygen = 8.5 - (3.82 + 0.43)
= 4.25 g
Moles of oxygen = 4.25 g/16 g/mol = 0.27 moles
We can now divide through by the lowest ratio;
C - 0.32 H - 0.43 O - 0.27
C - 1 H - 2 O - 1
The empirical formula is CH2O
The molecular formula is;
[12 + 2+ 16]n = 160
n = 160/30
n = 5
The molecular formula is;
C5H10O2
Learn more about molecular formula :https://brainly.com/question/20748250
#SPJ1

Explain two positive aspects of using methane recapture systems.
Answers
Answer:
Two positive aspects of using methane recapture systems are able to generate significant electricity. Another benefit is that the process of anaerobic digestion creates heat that can be used to warm buildings where animals are kept
Answer: The correct answer is;
Two positive aspects of using methane recapture systems include lowering the impact on greenhouse gasses and the production of energy. Methane is a very potent greenhouse gas that is contributing to global warming. As a result, the recapturing process reduces the methane impacts of global warming by reclaiming and reusing the gas for other purposes. Recaptured methane can be stored and used to generate electricity or used as fuel to power updated vehicles and other engines on the farm. The overall benefits from this combination are reducing impacts causing global warming and lower the cost of electricity or fuel on the farm.
Explanation: This answer has been confirmed correct.
A student studied the concept of order in the human body. Which
statement best summarizes how part of the human body is ordered?
1.cells are organized to form tissues
2. Tissues are organized to form cells.
3.organ systems work together to form tissues
4. Tissues work together to form organ systems.
5. Other
Answers
Answer:
1. cells are organized to form tissues
Explanation:
Cells are organized to form tissues is the statement best summarizes how part of the human body is ordered. Hence option 1 is correct.
What are cells?Cells are defined as the smallest component of living things, including biological tissues, that is capable of independent existence. The cell membrane, nucleus, and cytoplasm are the three major structural components of a cell. A cell's membrane, which encloses it and regulates what enters and leaves it, controls the flow of chemicals.
Organs are formed from tissues, which are composed of cells. Organ systems, such as the skeletal and muscular systems, are groups of organs. Cells, tissues, organs, and organ systems make up the several levels of organization in the human body. The human body, which is made up of countless billions of cells, is a marvel of structure and functionality.
Thus, cells are organized to form tissues is the statement best summarizes how part of the human body is ordered. Hence option 1 is correct.
To learn more about cells, refer to the link below:
https://brainly.com/question/30046049
#SPJ6
An atom with5 protons 6 neutrons and 5 electrons has an atomic mass of Amy
Answers
Answer: The number of neutrons for a given element is the only number that can change and still have the identity of the element stay the same, (because the atomic number is the number of protons). In this case the mass number would be 11.
Hope this helps...... Stay safe and have a Merry Christmas!!!!!! :D
Which of the following elements has the strongest ionization energy: Mg or Cl? Define ionization
energy and explain why based on the attractive force between protons and electrons.
Answers
Answer:
Cl
Explanation:
The element Cl will have the strongest ionization energy from the given choices. Most non-metals have higher ionization energy compared to metals.
Ionization energy is the energy required to remove the most loosely held electron from the gaseous phase of an atom.
As you go from left to right on the periodic table, it increases progressiveFrom top to bottom, the ionization energy reduces significantly. The attractive force between the protons in the nucleus and the electrons plays a very important role. In metals, they have very large atomic radius, the attractive force on the outer electrons is very weak. This is not the case in non-metalsFor each question, determine the type of reaction, then balance the reaction.
Problems 1-4: 2 points each
1.
Al +
H, Te →
Al, Tez +
H
a. Type:
2.
_C12H26 +
O2 →
H2O +
CO2
a. Type:
3.
Au +
CuSO4 →
Cu +
AuSO4
a. Type:
4.
H₂O →
H2 +
a. Type:

Answers
Answer:
i know the answer but i dont have time bro
what is gravity and weight please answer
Answers
Weight is the force of gravity on an object. Weight depends on the strength of the gravitational field the object is in and the mass of the object. ... Mass is constant anywhere in the universe, whereas weight depends on the gravity where the object is present (Earth, the moon, Jupiter, etc.).
The combustion of ethylene proceeds by the reaction
C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (g)
When the rate of disappearance of C2H4 is 0.13 M s-1, the rate of appearance of CO2 is ________ M s-1.
Answers
According to 2011/Chemistry Reference Tables
A. The electronegativity value of Cobalt is higher than the electronegativity value of Nickel
B. The electronegativity value of nitrogen is higher than the electronegativity value of fluorine
C. The electronegativity value of calcium is lower than the electronegativity value of silicon
D. The electronegativity value of boron is lower than the electronegativity value of beryllium
Answers
Answer:
C
Explanation:
C. The electronegativity value of calcium is lower than the electronegativity value of silicon
is the only correct statement.
draw an energy diagram for an endothermic and exothermic reaction and label the diagram
Answers

The energy diagram for an endothermic reaction and exothermic reaction are attached in attachments below.
What are endothermic and exothermic reactions?An exothermic reaction is defined as a chemical reaction which involves release of energy in the form of light,heat .In these reactions, energy is transferred from system to surroundings rather than taking energy from surroundings into system as in endothermic reactions.
In an exothermic reaction,change in enthalpy is negative.Therefore, it can be inferred that net amount of energy which is required to start the exothermic reaction is less than the net amount which is released by the reaction.
An endothermic reaction is defined as a chemical reaction which is a thermodynamic process accompanied by an increase in enthalpy of the system.In this process, a system absorbs energy from the surroundings which is mainly thermal energy.
Learn more about endothermic and exothermic reactions,here:
https://brainly.com/question/10373907
#SPJ2


13.41g of Fe(NO3)2 is dissolved in 83.0mL of water. Calculate the molarity of the solution.
Answers
Answer:
whats the question in this
Explanation:
Which group is composed entirely of nonmetals?
Answers
You welcome and please give me brainiest!
Answer:
These are the nonmetals.

Why do we have to decide what to do in a day?
Answers
Answer:
otherwise you will be bord and have nothing to do
Explanation:
Otherwise, we would just be blobs and do nothing.
HELP !!!!!!! pls. with chemistry

Answers
Answer:
Qns no.3: it is not a correct answer
Explanation:
S has 6 outermost electrons and O has 6 outermost electrons, during bonding inorder to be stable their shouldn't be any unpaired electron in a structure. so 2 double bonds will be formed.

How do you solve real world problems that involve multiple percents?
Answers
Answer:
Percentages are used widely and in many different areas. For example, discounts in shops, bank interest rates, rates of inflation and many statistics in the media are expressed as percentages. Percentages are important for understanding the financial aspects of everyday life.
Explanation:
a calculator or be like me and ask otherslol