Answers
because convection is the movement caused within a fluid by the tendency of hotter and therefore less dense material to rise, and colder, denser material to sink under the influence of gravity, which consequently results in transfer of heat.
butter melting on warm pancakes is convection
The following information should be considered:
Since convection is the movement arise within a fluid by the tendency of hotter and therefore less dense material to rise,.Moroever, it is colder, denser material to sink under the impact of gravity, that leads in transfer of heat.learn more: https://brainly.com/question/2514933?referrer=searchResults
Related Questions
How do you identify a redox reaction?
Answers
Answer:
I think you have to calculate the oxidation number of each atom in the reaction.
Explanation:
Hope that helped! ^u^
You are given a solution containing a pair of enantiomers (A and B). Careful measurements show that the solution contains 98% A and 2% B. What is the ee of this solution
Answers
Answer:
ee = 96%
Explanation:
Enantiomeric excess, ee, is a way to express a mixture that is not enantiomerically pure. It is defined as 100 times the ratio between the differences of amounts of enantiomers and the total amunt. that is:
ee = |A-B|/ A+B * 100
ee = |98%-2%| / 98+2 * 100
ee = 96%Which of these sentences probably comes from a work of classic literature?) PLEASE HELP ILL GIVE YOU BRAINLEIST!
A. The greedy man was harsh as winter, and had never done a generous deed in his long life; he was a hermit.
B. The man who refused to share with those in need had never felt the warmth that comes from giving.
C. The most selfish man in town was one who lived alone and never had visitors of any sort, except maybe lawyers.
D. The miser was hard and sharp as flint, from which no steel had ever struck out generous fire; he was as solitary as an oyster.
Answers
The sentences that probably comes from a work of classic literature are:
The most selfish man in town was one who lived alone and never had visitors of any sort, except maybe lawyers. The miser was hard and sharp as flint, from which no steel had ever struck out generous fire; he was as solitary as an oyster.What types of books are regarded as classics?The great literary works of Greek, Roman, and other prehistoric civilizations are referred to as classical literature. Classical literature includes the writings of Homer, Ovid, and Sophocles. The phrase isn't just applicable to books. It can also incorporate other types of writing, such as epic, lyric, tragedy, comedy, and pastoral.
Classical literature enables us to take a profound plunge into the lives, worldviews, and mindsets of individuals we have never met, to travel to unfamiliar settings, and to comprehend eras we will never be able to experience ourselves.
Therefore, option C and D are correct.
Learn more about literature at:
https://brainly.com/question/20848481
#SPJ1
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
What is the purpose of the arrow in a chemical equation?
Answers
The arrow in a chemical equation represents the direction of the reaction. It indicates the conversion of reactants into products. The arrow points from the reactant side to the product side, symbolizing the flow of the reaction.
The purpose of the arrow is to visually represent the chemical transformation occurring in the reaction. It shows the relationship between the reactants and products and the direction in which the reaction proceeds. The arrow implies that the reactant molecules are being rearranged and transformed into new substances with different properties.
Chemical equations are used to describe the stoichiometry and balance of reactions. The arrow helps convey this information by illustrating the overall process taking place. It serves as a crucial element in understanding the reaction's composition, reaction conditions, and the substances involved.
Furthermore, the arrow also implies that the reaction can occur in both directions. In reversible reactions, the arrow can be represented as a double-headed arrow, indicating that the reaction can proceed in either direction depending on the conditions.
Know more about reversible reactions here:
https://brainly.com/question/21426719
#SPJ8
When balancing redox reactions under acidic conditions, hydrogen is balanced by adding: Select the correct answer below:
a. hydrogen gas
b. water molecules
c. hydrogen atoms
d. hydrogen ions
Answers
Answer:
water molecules
Explanation:
Redox reactions are carried out under acidic or basic conditions as the case may be.
If the reaction is carried out in an acid medium, then we must balance the hydrogen ions on the lefthand side of the reaction equation with water molecules on the righthand side of the reaction equation.
For instance, the equation for reduction of MnO4^- under acidic condition is shown below;
MnO4^-(aq) + 5e + 8H^+(aq) --------> Mn^2+(aq) + 4H2O(l)
Which statement is FALSE?
1) atoms in the same period have the same number of outer electrons
2) atoms in the same group behave in a similar manner during a chemical
reaction
3) the outer electrons are the electrons in the outer energy level of an atom
4) the number of outer electrons may be used to predict how an element would
react in a chemical reaction
Answers
Answer: I think the answer is 2.
Explanation:
consider the following chemical equilibrium: c(s) 2h2 now write an equation below that shows how to calculate from for this reaction at an absolute temperature . you can assume is comfortably above room temperature. if you include any common physical constants in your equation be sure you use their standard symbols, found in the aleks calculator.
Answers
At absolute temperature Kp and Kc can be calculated from the equation given as:
Kp = Kc/(R × T)
When Equilibrium occurs then the velocity of the formation of the products becomes equal to the velocity of the formation of the reactants at a reversible reaction. Due to this, the concentrations and also the partial pressures of the substances remains constant.
The equilibrium of the reaction can be characterized by the equilibrium constant, which can be calculated by the activity of the substances. After that for solids and also that the liquid water, the is activity is equal to 1. Now, for liquids and also those aqueous substances, it is also equal to the concentration, and that for gases, it can be equal to the concentration or that is the partial pressure.
Hence, keeping T constant and relation an be derived as Kp = Kc/(R × T), where R is the universal gas constant.
equilibrium
Learn more about equilibrium from the link given below.
https://brainly.com/question/24174547
#SPJ4
The density of a 20. 3 m ch3oh (methanol) solution is 0. 858 g/ml. What is the molality of this solution? h2o is the solvent.
Answers
Answer:
Explanation:
To calculate the molality of the solution, we first need to determine the number of moles of solute (CH3OH) in 1 kg of the solvent (H2O).
The density of the solution is given as 0.858 g/mL. This means that 1 L (1000 mL) of the solution has a mass of 858 g.
We can assume that the mass of the solvent (water) is 1000 g (i.e., 1 kg). Therefore, the mass of the solute (methanol) in 1 L of the solution is:
mass of CH3OH = mass of solution - mass of H2O
mass of CH3OH = 858 g - 1000 g = -142 g
The negative value indicates that there is no methanol in 1 L of pure water. Therefore, we need to use a smaller volume of the solution that contains a measurable amount of methanol. For example, let's consider 1 L of the solution:
mass of CH3OH in 1 L = 0.858 kg/L × 0.203 mol/L × 32.04 g/mol = 5.58 g
Now, we can calculate the number of moles of CH3OH in 1 kg of H2O:
moles of CH3OH = 5.58 g / 32.04 g/mol = 0.1745 mol
The molality of the solution is then:
molality = moles of solute / mass of solvent (in kg)
mass of 1 L of water = 1000 g / 1000 = 1 kg
molality = 0.1745 mol / 1 kg = 0.1745 m
Therefore, the molality of the 20.3 m CH3OH solution is 0.1745 m.
A solution is made by dissolving 12.50 g of NaOH, a strong base, in water to produce 2.0 liters of solution. What is the pH of this solution?
Answers
According to molar concentration and pH concept, the pH of this solution is 0.808.
Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.Substitution of values in formula gives, molarity= 12.5/40×1/2=0.156 M which is H+ concentration, thus pH=-log[H+]=-log[0.156]=0.808 M.
Learn more about molar concentration,here:
https://brainly.com/question/21841645
#SPJ1
A student makes a saturated sugar solution by dissolving sugar grains in warm water stirring and adding more sugar until it no longer dissolves the student then allows the solution to come to room temperature the student pours equal volumes of sugar solution into five Beakers the student places a string into each of the beakers with one end of the string hanging free outside of the glassware the student then places the bigger the five chambers of varying temperatures 5,10,15,20 and 25°C for two weeks at the end of the experiment a student student noticed crystals have formed in the string the student masses the amount of crystals that formed from the sugar solution on the string select the dependent variable in this experiment what process in the rock cycle the student is most likely modeling
Answers
Answer:
The answer is A
Explanation:
A student who performed the Benedict tests on potatoes is the subject of a case study. Benedict Regent's flavor is the first thing that comes to mind. Benedict's solution is used to complete Benedict's test.
What sugars by dissolving sugar grains in warm water?With the aid of a potato, the kids tasted. To do the test, she utilized potato and Benedict solution. When Benedict solution is present, the color is a vivid crimson orange, however after the student's taste, complicated sugar does not.
There is no need to display a vivid orange or red color in Benedict's answer. The pupil was holding a potato. The brilliant orange color in the solution was displayed as a result. The two sugars' inclusion is what gives the mixture its hue.
An experiment should have two distinct controls. A positive control should be one, and a negative control should be the other.
Therefore, The one who oversees positive controls is called a positive control.
Learn more about sugar here:
https://brainly.com/question/18835784
#SPJ2
What doesn’t change the resistance of a wire
Answers
The factor that doesn’t change the resistance of a wire is pressure. option A.
What is resistance of a wire?Resistance is a conductor's capacity to thwart the passage of current. It is controlled by the interplay of the applied voltage and the electric current passing through it. The amount of opposition any object applies to the flow of electric current is referred to as resistance.
The ohm, a unit of measurement for resistance, is represented by the Greek letter omega. According to Ohm's law, the voltage across two places is precisely proportional to the current flowing through a conductor between them.
Hence option A is correct.
Learn more about resistance at
https://brainly.com/question/17563681
#SPJ1
missing part;
The pressure
The length of the resistor.
The thickness of the resistor.
The temperature of the conductor.
2 examples of metal’s catalytic reaction
Answers
Answer:
Example 1
palladium(II) nitrate,
Example 2
Metal catalysts such as Fe, Ni, Mo, and Co are routinely used in the manufacture of CNMs.
Explanation
The three metals used in catalytic converters — rhodium, platinum and palladium — are part of a category known as platinum group metals, or PGMs, which are known for their catalytic properties.
How many moles of C₂H5OH
are there in 45.0 mL of 0.250 M
C₂H5OH?
Answers
Answer:
no. of moles = 0.0113
Explanation:
To calculate the number of moles of a substance given its concentration and volume, we can use the following formula:
\(\boxed{\mathrm{No. \ of \ moles = Concentration \times \frac{Volume}{1000}}}\),
where concentration is mole/dm³ or M, and volume is in cm³ or ml.
In this question, we are told that:
• concentration = 0.250 M
• volume = 45.0 ml
Substituting these values into the formula above, we get:
No. of moles = \(0.250 \times \frac{45.0}{1000}\)
= \(0.250 \times 0.045\)
= 0.01125
\(\approx\) 0.0113 (3 s.f.)
Therefore, the number of moles of C₂H₅OH is 0.0113.
The 0.0112 moles of C2H5OH are present in the 0.250 M solution of C2H5OH.
What is a mole?
Mole is used as a concentration unit in chemistry terms. One mole is equal to the mass of the given compound which is divided by the molar mass of the same compound.
Given:
M= 0.250M ( M stands for molarity)
Volume = 45.0 ml
The molar mass of C2H5OH = 46.07 g/mol
Now, we use molarity formula M = Moles of the substance/volume in liter
Let say moles = x
Units of M = mol/L
Therefore, units of volume need to be converted in to Liters = 1L = 1000ml
By using molarity equation, X= 45.0*0.250/1000 = 0.0112 moles
Hence, the required number of moles of C2H5OH = 0.0112 moles.
To learn more about mole concept Check the given link
https://brainly.com/question/15356425
#SPJ9
In the chemical equation Cu + 2AgNO3 = 2Ag +Cu(NO3)2, why is Cu(NO3)2 that and not CuNO3? Can Copper (i) Nitrate exist? Why or why not?
Answers
NO. One displacement reaction has occurred here. One element can substitute for another.
The majority of precipitates are created by twofold displacement processes, in which reactant ions swap locations to create new products, one of which will precipitate out of the solution.
What does displacement response refer to?A displacement reaction occurs when an atom or group of atoms in a molecule are replaced by another atom. For instance, when iron is introduced to a solution of copper sulphate, the copper metal is replaced. A-C-B = A + B-C. When A is more reactive than B, the preceding equation holds.
There are two displacement reactions as examples: iron sulphate is the result of the interaction between iron and copper sulphate. Iron replaces copper in this situation because it is more reactive than copper. the process by which zinc reacts with iron sulphate to produce zinc sulphate as a byproduct.
Displacement reactions come in two varieties: single displacement reactions and double displacement reactions.
learn more about displacement reaction refer
https://brainly.com/question/7959057
#SPJ13
Write balanced, net ionic equations for the following precipitation, acidn base, or gasn forming reactions. Include states of matter. Hint: You should write the molecular equation first to predict the products. a) mixing aqueous solutions of iron (III) chloride and lithium sulfide b) mixing aqueous solutions of sodium acetate and ammonium phosphate c) mixing aqueous solutions of perchloric acid and potassium hydroxide d) mixing aqueous solutions of ammonia and nitric acid e) mixing aqueous solutions of nitrous acid and sodium hydroxide f) adding aqueous hydroiodic acid to solid calcium carbonate g) Problems c) through f) are acid- base reactions. Comment on the differences in the net ionic equations for these reactions.
Answers
Answer:
Explanation:
a ) 2FeCl₃ + 3Li₂S = Fe₂S₃ ( s ) + 6 LiCl
2Fe⁺³ + 6Li ⁻ + 6Cl⁻ + 3S⁻² = 6Li + 6Cl⁻ + Fe₂S₃ ( s )
b )
3CH₃COONa +( NH₄)₃PO₄ = 3CH₃COONH₄ + Na₃PO₄
3CH₃COO + 3Na⁺ + 3NH₄⁻ + PO₄⁺³ = 3CH₃COO⁻ +3NH₄⁺ + Na₃PO₄
c )
HClO₄ + KOH = kClO₄ + H₂O
H ⁺ + ClO₄⁻ + K⁺ + OH⁻ = k⁺ ClO₄⁻ + H₂O
d )
NH₄OH + HNO₃ = NH₄NO₃ + H₂O
NH₄⁺ + OH⁻ + H⁺ + NO₃⁻ = NH₄⁺ + NO₃⁻ + H₂O
e )
HNO₂ + KOH = KNO₂ + H₂O
H⁺ + NO₂⁻ + K⁺ + OH⁻ = K⁺ + NO₂⁻ + H₂O
f ) HIO₃ + CaCO₃ ( s ) = Ca( IO₃ )₂ + H₂CO₃
H⁺ + IO₃⁻ + CaCO₃ ( s ) = Ca( IO₃ )₂ + H₂CO₃
g )
c ) is strong acid and strong base
d ) is weak base and strong acid
e ) weak acid and strong base
f ) Strong acid and basic salt
a substance has a known density of 1.76 g/mL and you have found a flask with 147 mL of the substance inside of it. what is the mass of the substance you found
Answers
Answer:
The answer is
258.72 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume of substance = 147 mL
density = 1.76 g/mL
The mass of the substance is
mass = 1.76 × 147
We have the final answer as
258.72 gHope this helps you
Convert a flow rate of 3.15 x 10^-5 kL.hr-1 to µL.s-1.
Answers
According to the given statement, A flow rate of 3.15 x 10^-5 kL.hr⁻¹ is equivalent to 8.75 µL.s⁻¹. To convert kL.hr⁻¹ to µL.s⁻¹, we can use the steps: (Given below)
What is flow rate and how is it measured?liquids flow is the amount of liquids that moves in a given amount of time. When measuring the flow of water supplies, different units are frequently used, including cubic feet per second (cfs), cubic meters per second (cms), gallons per minute (gpm), and others.
⇒ Convert kiloliters (kL) to liters (L) by multiplying by 1000.
⇒ Convert hours (hr) to seconds (s) by multiplying by 3600.
⇒ Convert liters per hour (L.hr⁻¹) to microliters per second (µL.s⁻¹) by dividing by 3.6.
Using these steps, we get:
3.15 x 10^-5 kL.hr⁻¹ = 3.15 x 10^-5 x 1000 L.hr⁻¹ = 0.0315 L.hr⁻¹
0.0315 L.hr⁻¹ = 0.0315 / 3600 L.s⁻¹ = 8.75 x 10^-6 L.s⁻¹
8.75 x 10^-6 L.s⁻¹ = 8.75 x 10^-6 x 10^6 µL.s⁻¹ = 8.75 µL.s⁻¹
Therefore, a flow rate of 3.15 x 10^-5 kL.hr⁻¹ is equivalent to 8.75 µL.s⁻¹.
To know more about flow rate visit :
https://brainly.com/question/1154328
#SPJ1
A cylinder of Krypton has contains 17 L of Ar at 22.8 atm and 112 degrees celsisus. How many moles are in the cylinder?
Answers
Given :
A cylinder of Krypton has contains 17 L of Ar at 22.8 atm and 112 degrees Celsius.
To Find :
How many moles are in the cylinder.
Solution :
We know, by ideal gas equation :
\(PV = nRT\\\\n = \dfrac{PV}{RT}\)
Here, R is gas constant and \(R = 8.205 \times 10^{-5} \ m^3\ atm\ K^{-1} mol^{-1}\)
Converting all given in required units and putting in above equation, we get :
\(n = \dfrac{P\times V}{ R \times T}\\\\n = \dfrac{22.8 \times 0.017}{8.205\times (112+273)}\ moles\\\\n = 1.22 \times 10^{-4} \ moles\)
Hence, this is the required solution.
that dissolve in water and has salty taste
Answers
Answer:
it is salt an I right or not
Table salt
(NaCl)
This is the answer
When some solid ammonium nitrate was dissolved in water the temperature decreased from 22 oC to 3 oC. What can be deduced from this observation?
The dissolving is endothermic and ∆H is positive. The dissolving is endothermic and ∆H is negative. The dissolving is exothermic and ∆H is positive. The dissolving is exothermic and ∆H is negative.
Which is a correct statement about an endothermic reaction?
A. The bonds in the reactants are stronger than in the products and ∆H is positive.
B. The bonds in the products are stronger than in the reactants and ∆H is positive.
C. The bonds in the reactants are stronger than in the products and ∆H is negative
D. The bonds in the products are stronger than in the reactants and ∆H is negative.
Which is a correct statement about the following() enthalpy level diagram of a reaction? A. The reaction is exothermic and △H is positive.
E. The reaction is exothermic and △H is negative.
F. The reaction is endothermic and △H is positive.
G. The reaction is endothermic and △H is negative.
Which statements are correct for all exothermic reactions?
I. The products are more stable than the reactants.
II. The bonds in the products are stronger than the bonds in the reactants
III. The enthalpy of the products is less than the enthalpy of the reactants
A. I and II only
B. I and III only
C. II and III only
D. I, II and III

Answers
Answer:
1. The dissolving is endothermic and deltaH is positive
2. A. The bonds in the reactants are stronger than in the products and deltaH is positive
3. I can't see the diagram so I don't know the answer (it's a glitch on my part not yours, my school district blocks images automatically on their rented chromebooks)
4. A. I, and II only
Explanation:
When we measure the temperature of a solution, and we notice that the temperature of the water is decreasing, then this is an endothermic reaction..
The dissolved solute (the "invisible" ions) are absorbing the heat, causing the water around it to be colder.. **Heat moves from hot to cold, so the water is losing it's heat and the dissolving (dissolved solute) is gaining heat, meaning that it is Endothermic.
When a reaction is endothermic, the energy that is required to break bonds and attractive forces is greater than the energy required to form bonds and attractive forces. This means that the bonds in the reactants are stronger than in the products and deltaH is positive. Since we know that the energy in the reactants is greater, there is more heat in the reactants. This is also why the dissolved solute would be gaining heat.. If the energy of the reactants is stronger, when it breaks, it will release a lot of energy that the dissolved solute will be absorbing.
Think, heat (q, released or absorbed) is measured in Joules, the higher the Joules, the hotter something will be. So, if the heat (q) is negative, that represents the amount of Joules (heat) being released. If it is positive, that is the amount being absorbed. So, if heat is being absorbed then deltaH is positive.
If the diagram shows the products being at a higher energy level than the reactants, then the diagram shows an endothermic reaction.. If we see that the energy from the products is greater, than the energy of the system increased as it absorbed energy from its surroundings.
If the diagrams shows the reactants being at a higher energy level than the products, then the diagram shows an exothermic reaction.. Think of it this way, if we see that the energy from the reactants was greater, than the energy of the system was originally greater than the surroundings, but now (observing the products), it is not, as energy was lost to the surroundings, making this an exothermic reaction.
For exothermic reactions, the energy that is required to break bonds and attractive forces is less than the energy required to form bonds and attractive forces.. This means that energy in the products are greater, so as these bonds are broken and energy is released, the surroundings gain energy/heat. This means that the energy is favorable (more stable), the more negative our potential energy (energy of the system) is, the more favorable our process is.
SCl6, K2S, ClO2, N2O, NaO2 Which of the following compounds are formed by ionic bonding?
Answers
Answer:
• SCl6 [ Sulphur hexachloride ]
• Na2O [ Sodium oxide ]
Or:
• Na2O2 [ Sodium peroxide ]
Explanation:
→ Ionic bonding or electrovalent bonding is the type of bonding between a metal and a non metal, where a metal loses its Outermost electrons to the non metal in order to gain stability.
\(.\)

The atomic number of an atom is
A. The mass of the atom.
B. The number of protons added to the number of neutrons in the nucleus.
C. The number of protons in the nucleus.
D. Negatively charged.
Answers
Answer:
B. the number of protons added to the number of neutrons in the nucleus.
Explanation:
Sana makatulong
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
Why is the classification species not considered a group? (1 point)
O Each species is a separate type of organism.
O Each species is an individual organism.
O Each species lacks the characteristics of the levels above.
O Each species shares characteristics with other species.
Answers
Each species is a separate type of organism.
A species is a group of creatures that share similar traits. The same species of organisms are capable of sexual reproduction as well as interbreeding and producing fertile offspring. It is a fundamental unit of taxonomy and classification.The system is divided into seven categories: Kingdom, Phylum or Division, Class, Order, Family, Genus, and Species. Kingdom is the most inclusive category.In a group, many types of an organism can be included even if they do not share the same traits. But species is a group of organisms that share similar traits.For example, human beings are species as they are all alike in physical features, way of reproduction, etc. But the animal is considered a group because it included a variety of living beings.Therefore, Each species is not considered a group.
Learn more about taxonomy here:
https://brainly.com/question/1304906
#SPJ9
In an ionic bond, how does a sulfur atom achieve an octet of electrons?
By gaining 2 electrons
By gaining 6 electrons
By losing 2 electrons
By losing 6 electrons
Answers
PLEASE HELP I DO NOT UNDERSTAND ANY OF THIS
Mass (g)
crucible + lid 31.064
crucible + lid + Mg ribbon 31.634
crucible + lid + final solids 31.970
Calculations:
1. Calculate the mass of magnesium that was added to the crucible.
2. Calculate the number of moles of magnesium that were added to the crucible.
3. Calculate the mass of oxygen that was added to the crucible using the mass of the solid MgO product.
4. Calculate the number of moles of O atoms that were added to the crucible.
5. Using your answers to questions 2 and 4, write the empirical formula for MgxOy. (i.e. determine the values of x and y in the empirical formula). Reduce the ratio of x:y to whole numbers.
6. Based on the charge of the magnesium and oxygen ions, did you measure the expected ratio of magnesium to oxygen ions in magnesium oxide?
Answers
Answer:
1.31.634-31.064=570g
2. mass in grams /RAM=570g/24=23.8 moles
3. 31.970-31.634=336g
4. no. of moles=mass in grams /RAM=336g/16*2=10.5 moles
5.Mg1O1
explanation:
A piece of dry ice at -76°C begins to sublimate (solid to
gas) when placed at the counter at room temperature. How
many bars are in the final phase energy (Eph)?
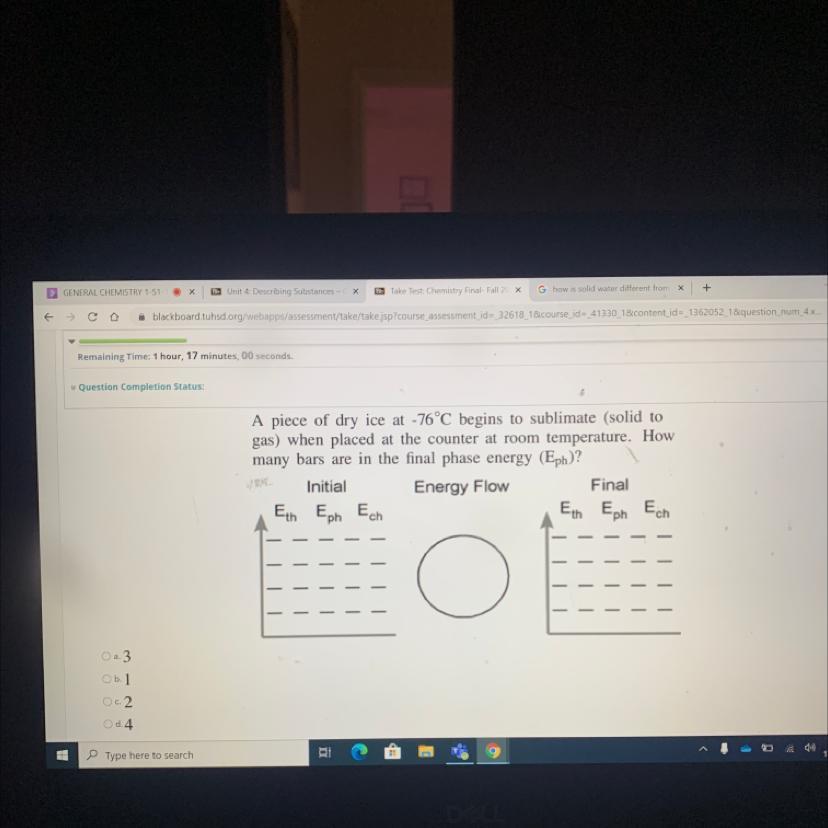
Answers
Answer:
D
Explanation:
4 bars
1. Why do magnets repel each other?
2. Why do magnets attract some metal objects?
3. What happens if a magnet breaks in ½?
4. How do you make a piece of metal magnetic?
Please please help