Is hydrogen a metal or a nonmetal? How many valence electrons does a hydrogen atom have?
Answers
Answer:
hydrogen is a non-metal, and has one valence electron.
Explanation:
Hope this helps!!
Hydrogen shows properties of both metals and nonmetals. However, hydrogen is placed with the alkali metals since it contains one valence electrons.
What is hydrogen ?Hydrogen is the first element in the periodic table. It has only one electron and it is the valence electron of hydrogen. Therefore, hydrogen is placed in the first group of periodic table.
Hydrogen needs one more electrons to become stable. The 17th group elements halogens also have a valency of one. Hence, hydrogen shows some chemical similarities with halogens also.
Mostly hydrogen forms covalent bonds like non-metals and in rare cases, hydrogen forms ionic bonds by donating its electron. Therefore, hydrogen is mostly considered as a non-metal.
Find more on hydrogen;
https://brainly.com/question/11837837
#SPJ2
Related Questions
be sure to answer all parts. determine a detailed mechanism for the chlorination of benzene using cl2 and fecl3.
Answers
The chlorination of benzene using Cl2 and FeCl3 involves the generation of an electrophilic chlorine species, its attack on the benzene ring, and subsequent regeneration of the aromatic system through proton transfer.
The chlorination of benzene using Cl2 and FeCl3 proceeds through an electrophilic aromatic substitution mechanism. Initially, FeCl3 acts as a Lewis acid catalyst and interacts with Cl2 to generate a strong electrophile, Cl+. The FeCl3 complex helps in polarizing the chlorine molecule and facilitating the formation of the electrophilic species.
In the next step, the electrophilic chlorine species (Cl+) attacks the benzene ring, targeting one of the hydrogen atoms attached to a carbon atom. The pi electrons of the benzene ring act as a nucleophile, attacking the electron-deficient chlorine atom. This results in the formation of a sigma complex intermediate, where the chlorine atom has replaced a hydrogen atom on the benzene ring.
Finally, the FeCl3 catalyst assists in regenerating the aromaticity of the benzene ring by abstracting a proton from the sigma complex intermediate. This proton transfer step generates HCl and restores the aromaticity of the substituted benzene ring.
Learn more about chlorination here:
https://brainly.com/question/32227367
#SPJ11
In a garden, an ant walked past an earthworm that was moving at 7.2 millimeters per second. When it passed the worm, the ant had walked 458millimeters directly toward a plant at a constant velocity. It took the ant 10.0seconds to walk that distance. What was the ant's velocity?
Answers
Answer:
45.8 millimeters per second.
Explanation:
To find the ant's velocity, we need to first find the distance it traveled and the time it took to travel that distance.
The distance the ant traveled is 458 millimeters, and the time it took to travel that distance is 10.0 seconds.
To find the ant's velocity, we divide the distance it traveled by the time it took to travel that distance.
This gives us a velocity of 458 millimeters / 10.0 seconds = 45.8 millimeters per second.
_______ are events that disrupt the stability of a whole ecosystem.
A.Resources
B.Disturbances
C.Species

Answers
Volcanoes can be destructive LOCALLY causing all of the following immediate effects EXCEPT:
O New growth in forests
O Personal damage
O Lack of breathable air
O Disruption of clean water
O Death
Answers
Answer:
New growth of trees is an exception
Explanation:
when volcanoes erupt, they release hot magma that is destructive to the environment causing personal damage, lack of breathable air and death.
heat produce cannot in any way help in growth of trees
60 points one of the questions im stuck on
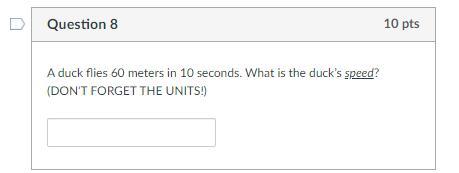
Answers
Answer:
6 m/s is the answer.
Explanation:
speed = distance/time
(please mark me brainliest if you can)
Answer:
6 meters per second
Explanation:
they fly 60 meters in 10 seconds. So to find how many meters they fly in one second, we divide by 10.
60m/10secs = 6 m/s
Whose personal story did you read (if you read several, pick your favorite)? What was the most interesting or eye-opening part of that person's story?
Answers
What is the percent sodium in sodium chloride?
Answers
The total mass of sodium chloride is 58.44 g/mol.
The mass of sodium is 22.99 g/mol.
To find the percent sodium in sodium chloride can be found by dividing the amounts.
\(\frac{22.99}{58.44}\approx0.39\)Therefore, the percent sodium is 39%.
What is the reaction type
2 Mgl2+ Mn(SO3)2 → 2 MgSO3 + Mnl4
Answers
Answer:
The reaction type is double displacement
products for combustion
Answers
Answer:
Carbon Dioxide
Carbon Monoxide
Sulfur Dioxide
Nitrogen Oxides
Lead
Particulate Matter
Explanation:
Which commercial technology commonly uses plasmas? a radio a race car a television a microwave oven
Answers
Answer:
c
Explanation:
The commercial technology commonly uses plasma is a television. The correct option is c.
What is commercial technology?Commercial technology is any technology that is employed in the business world, including electric power, radio, television, phones, and other similar devices. These items are employed commercially in a variety of fields. An electrical gadget called television is used to watch entertainment.
Plasma is a substance found in televisions; these gas-filled pockets receive electricity to transform into plasma screens. The UV rays that these plasmas then emit create a picture as they pass through the phosphorus cells.
Thus, the correct option is c. a television, regarding a commercial technology that commonly uses plasmas.
Learn more about commercial technology, here:
https://brainly.com/question/17009267
#SPJ5
The table below contains the bond dissociation energies for common bonds.
Bond Dissociation energy
(kJ/mol )
C−C 350
C=C 611
C−H 410
C−O 350
C=O 799
O−O 180
O=O 498
H−O 460
Calculate the bond dissociation energy for the breaking of all the bonds in a mole of methane, CH4.
Answers
The bond dissociation energy for breaking all the bonds in a mole of methane (CH4) is 1640 kJ/mol.
To calculate the bond dissociation energy for breaking all the bonds in a mole of methane (CH4), you'll need to consider the bond dissociation energies for the C-H bond, which is provided in the table.
The methane molecule (CH4) has four C-H bonds. According to the table, the bond dissociation energy for a single C-H bond is 410 kJ/mol.
Step 1: Calculate the energy needed to break one molecule of methane by breaking all four C-H bonds:
Energy = 4 (C-H bonds) * 410 kJ/mol (bond dissociation energy for C-H)
Energy = 1640 kJ/mol
Step 2: Calculate the energy needed to break all the bonds in a mole of methane:
Energy = 1 mole of CH4 * 1640 kJ/mol
Therefore, the bond dissociation energy for breaking all the bonds in a mole of methane (CH4) is 1640 kJ/mol.
Learn more about bond dissociation energy at https://brainly.com/question/24301293
#SPJ11
Can You Tell?
Pepsinogen is released in the stomach. Why can't it digest stomach walls?
Answers
Answer:
Specific cells within the gastric lining, known as chief cells, release pepsin in an inactive form, or zymogen form, called pepsinogen. By doing so, the stomach prevents the auto-digestion of protective proteins in the lining of the digestive tract.
Explanation:
Make sure to edit so you don't get copy-writed
Which of the following outer electron configurations could belong to a metalloid? Check all that apply. ns2np6, ns2np2, ns2np5, ns2
Answers
A metalloid is an element that has properties of both metals and nonmetals. The outer electron configuration of a metalloid can vary depending on the specific element.
However, some common outer electron configurations for metalloids include ns2np2, ns2np5, and ns2.
The ns2np2 configuration is typically found in elements such as boron and silicon. The ns2np5 configuration is found in elements such as arsenic and antimony. The ns2 configuration is found in elements such as germanium.
It's important to note that not all elements with these configurations are metalloids. For example, oxygen and sulfur have ns2np4 and ns2np5 configurations, respectively, but are considered nonmetals.
In summary, the outer electron configurations that could belong to a metalloid include ns2np2, ns2np5, and ns2.
To know more about metalloid. please visit.....
brainly.com/question/29379145
#SPJ11
.
Your brother is curious about what your compound is shaped like and he wants you to draw it for him. What formula should you use that will give him a basic idea of how it is arranged?
Baby formula
Molecular formula
Structural formula
Empirical formula
Answers
To give a basic idea of the arrangement of a compound, the best formula to use is the structural formula. The structural formula shows the arrangement of atoms and the bonds between them, which gives a visual representation of the molecule's shape.
The molecular formula only provides information on the number and type of atoms in the molecule. The empirical formula provides the simplest ratio of atoms in the molecule, but does not show the arrangement or connectivity of the atoms. The baby formula is a completely unrelated term and is not relevant to chemistry or molecule representation.
Therefore, to show the arrangement of atoms and bonds in a molecule, the structural formula is the best choice.
For more questions like Chemistry visit the link below:
https://brainly.com/question/30958745
#SPJ11
chemistry help please!!

Answers
Answer:
no so boring........................
Which of the following is an unsafe act that might be found with ladders? Select one: a. Broken rungs b. Cracked rails c. Overextended ladders d. Overreaching
Answers
The unsafe act that might be found with ladders is d. Overreaching.
Overreaching refers to extending one's reach beyond a safe and stable position while standing on a ladder. This can lead to a loss of balance and stability, increasing the risk of falls and accidents.
It is important to use ladders properly and maintain a stable position within the ladder's recommended reach limits. Overreaching can put undue stress on the ladder, causing it to become unstable or tip over.
To ensure safety when using ladders, it is crucial to follow proper ladder usage guidelines, including maintaining three points of contact, using a ladder of appropriate height, and avoiding overreaching or leaning to the side.
To know more about the ladders refer here :
https://brainly.com/question/14975538#
#SPJ11
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work
HELP
1) A 400g sample of alcohol (c = 2.43 J/g°C) at 16°C is mixed with 400g
of water (c = 4.19 J/g°C) at 85°C. What is the final temperature of the
mixture?
Answers
Answer:
Explanation:
Given data:
Mass of alcohol = 400 g
Specific heat capacity of alcohol = 2.43 J/g°C
Initial temperature of alcohol = 16°C
Mass of water = 400 g
Specific heat capacity of water = 4.19 J/g°C
Initial temperature of water = 85°C
Final temperature of mixture = ?
Solution:
Equation:
m₁c₁ (T₂-T₁ ) = m₂c₂(T₂-T₁)
by putting values,
400 × 4.19 × (T₂ - 85°C) = 400 × 2.43 × (T₂ - 16°C)
1676 × (T₂ - 85°C) = 972 × (T₂ - 16°C)
When the [CO2] and [H2CO3] are both horizontal lines, the rate of the forward reaction is
the rate of the reverse reaction
faster than
slower than
the same as
Answers
When \(CO_{2}\) and \(H_{2} CO_{3}\) are both horizontal lines, the rate of the forward reaction is the same as the rate of the reverse reaction. The reaction is occurring at equilibrium, with no net change in the concentrations of reactants and products over time.
When the concentration of carbon dioxide \(CO_{2}\) and the concentration of carbonic acid \(H_{2} CO_{3}\) are both horizontal lines, it indicates that their concentrations remain constant over time. In such a scenario, the rate of the forward reaction is the same as the rate of the reverse reaction. A horizontal line on a concentration-time graph suggests that the concentrations of the reactants and products are not changing, implying that the reaction has reached equilibrium. At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction. This is a fundamental principle of chemical equilibrium, described by the principle of microscopic reversibility.
For more question on reaction
https://brainly.com/question/25769000
#SPJ8
true or false Given the recipe: 2 cups flour + 1 egg + 3 oz blueberries → 4 muffins
If you have 5 cups of flour, 3 eggs and plenty of blueberries, the limiting reactant is the eggs.
Answers
True. The limiting reactant is the reactant that is completely consumed in a chemical reaction, limiting the amount of product that can be formed. In this case, the recipe calls for 2 cups of flour and 1 egg to make 4 muffins.
Therefore, if you have 5 cups of flour and plenty of blueberries, the flour is not limiting the reaction since you have more than enough. However, you only have 3 eggs, which is less than the amount needed to make 4 muffins according to the recipe.
This means that the eggs are the limiting reactant in this scenario, as you cannot make more muffins than the amount of eggs you have. Therefore, you will be limited to making only 3 muffins with the given amount of eggs, even if you have excess flour and blueberries.
To know more about limiting reactant, refer here:
https://brainly.com/question/14225536#
#SPJ11
Help me about it please

Answers
Answer:
4 atoms of Hydrogen
1 atom of Sulphur
4 atoms of Oxygen
hope it helps you
make me brainliest plz
y'all need to help me please

Answers
Explanation:
2. The periodic table of elements is a tabular representation of chemical elements, organized on the basis of their atomic numbers
3. a. Mg
c.Fe
b.K
d. Cu
4. a.carbon
b.chlorine
c.aluminum
d.strontium
5. a. 5 group 18 element
b. 5 group 13 element
d. 5 group 17 element
c. 5 group 1 element
6. a. 3 group
b. 4 group
c. 5 group
d.4 group
7. I don't know
and.... you're welcome :))❤️


help me pls. the answer i got is 42.78 but it keeps saying it’s wrong

Answers
Answer: try 42.8
Explanation: you might have to round to 42.8 because of the significant figures
oxygen gas reacts with aqueous hydrazine (n2h4) to produce aqueous hydrogen peroxide and nitrogen gas. when 18.5 g of o2 reacts completely with excess n2h4 , the reaction produces what mass of n2?
Answers
The reaction of 18.5 g of O₂ with excess N₂H₄ produces approximately 16.2 g of N₂.
To determine the mass of N₂ produced when 18.5 g of O₂ reacts completely with excess N₂H₄, we need to use the balanced chemical equation and the molar masses of the compounds involved.
The balanced chemical equation for the reaction is as follows:
2 N₂H₄ + O₂ → 2 H₂O₂ + N₂
The molar mass of O₂ is approximately 32 g/mol, and the molar mass of N₂ is approximately 28 g/mol.
First, we calculate the number of moles of O₂:
Moles of O₂ = Mass of O₂ / Molar mass of O₂
Moles of O₂ = 18.5 g / 32 g/mol
Moles of O₂ ≈ 0.578 moles
According to the stoichiometry of the balanced equation, 1 mole of O₂ reacts to produce 1 mole of N₂. Therefore, the number of moles of N₂ produced is also approximately 0.578 moles.
Finally, we calculate the mass of N₂:
Mass of N₂ = Moles of N₂ × Molar mass of N₂
Mass of N₂ = 0.578 moles × 28 g/mol
Mass of N₂ ≈ 16.2 g
learn more about moles here:
https://brainly.com/question/28239680
#SPJ4
HCN is a monoprotic weak acid with a K, value of 4.90 x 10^-10. Calculate the pH of a 9.00 x 10-6 M solution of this acid ignoring the effects of the autoprotolysis of water. pH = ___Calculate the pH of the same acid at the same concentration taking into account the effects of the autoprotolysis of water. pH= ___
Answers
The pH of a 9.00 x 10^-6 M solution of HCN ignoring the effects of the autoprotolysis of water is 5.17.
The pH of a 9.00 x 10^-6 M solution of HCN ignoring the effects of the autoprotolysis of water is 5.17.
Explanation
The pH of a 9.00 x 10^-6 M solution of HCN is 5.17, ignoring the effects of the autoprotolysis of water. When taking into account the effects of the autoprotolysis of water, the pH of the same acid at the same concentration is 5.14.
To calculate the pH of a 9.00 x 10^-6 M solution of HCN ignoring the effects of the autoprotolysis of water
The dissociation reaction for HCN is:
HCN + H2O ⇌ H3O+ + CN-
According to the question, K value of the HCN is 4.90 x 10^-10. For weak acids, we can use the following expression to calculate the pH of the acid:
[H3O+] = √(Ka[C])
Here, Ka = K of the acid, C = concentration of the acid. We can substitute the given values of K and C in the equation above to calculate the [H3O+] concentration as:
[H3O+] = √((4.90 x 10^-10)(9.00 x 10^-6))= 2.07 x 10^-8M
We can now calculate the pH of the solution as:
pH = -log[H3O+]= -log (2.07 x 10^-8)= 5.17
Therefore, the pH of a 9.00 x 10^-6 M solution of HCN ignoring the effects of the autoprotolysis of water is 5.17.
How to calculate the pH of the same acid at the same concentration taking into account the effects of the autoprotolysis of water?
The auto-ionization of water can be written as:
2H2O ⇌ H3O+ + OH-
Since, [H3O+] concentration is due to the dissociation of HCN only, we can use the same expression we used above to calculate the [H3O+] concentration.
[H3O+] = √((4.90 x 10^-10)(9.00 x 10^-6))= 2.07 x 10^-8M
We can now calculate the pOH of the solution as:
pOH = -log[OH-]
= -log(Kw/[H3O+])
= -log(1.0 x 10^-14/[2.07 x 10^-8])
= 8.14pH
pH = 14.00 - pOH
= 14.00 - 8.14
= 5.86
Therefore, the pH of a 9.00 x 10^-6 M solution of HCN ignoring the effects of the autoprotolysis of water is 5.17.
"autoprotolysis of water", https://brainly.com/question/31055184
#SPJ11
A chemical reaction combines the metal sodium (NA) and the gas chlorine (cl2) to form sodium chloride, commonly known as table salt (nacl) why does this produce have different properties from the reactants
Answers
When a chemical reaction happens, the atoms in the reactants are rearranged to create compounds with various chemical characteristics. Although identical atoms are present in both the reactants and the products, sodium chloride was produced by rearranging the atoms in the reactants. The reactants lacked sodium chloride.
Sodium is a soft, silver-colored metal that may be sliced with a knife. With water, pure sodium metal reacts violently (and even explosively), creating sodium hydroxide, hydrogen gas, and heat.
2Na(s) + 2H₂O(l) ⟶ 2NaOH(aq) + H₂(g)
Chlorine is a poisonous yellow-green gas with a pungent smell.
But when sodium and chlorine combine, they create sodium chloride, or table salt, which is known to practically everyone in the world.
2Na(s) + Cl₂(g) ⟶ 2NaCl(s)
Visit the link below to learn more about the balanced equations:
brainly.com/question/27649738
CH4 + 2O + CO2 + 2 H2O
If 9.65 mole of methane reacts with oxygen to produce carbon dioxide and water, what mass of water is produced?
Answers
Answer:
347.4 (19.3 mole)
Explanation:
CH4 + 2O2 = CO2 + 2H2O
1(mole) - 9.65(mole)
2(mole) - x ⇒x = 9.65*2=19.3
m=n*M ⇒ m= 19.3*(1*2+16)= 19.3*18=347.4(g)
I need some help with chem, how do you know whether the reaction is endothermic or exothermic? Pls provide examples.
Thank you!!
Answers
Answer:
Basing on explanations below;
1. Endothermic reactions have a positive enthalpy while Exothermic reactions have a negative enthalpy.
2. Endothermic reactions take place in high temperatures while Exothermic reactions take place in low temperatures.
How is the process of mitosis essential to the survival of an organism?
Answers
Answer:
Explanation:
Mitosis is crucial to this process. Mitosis is the reason we can grow, heal wounds, and replace damaged cells. Mitosis is also important in organisms which reproduce asexually: this is the only way that these cells can reproduce. This is the one key process that sustains populations of asexual organisms.Jul 22, 2020
Mitosis has an impact on life because it regulates the growth and regeneration of billions of cells within the body.
Absent of mitosis, cell material would degrade quickly and cease to function correctly. We can develop, repair wounds, and replace damaged cells because of mitosis. Mitosis is a vital step for an organism's existence. Mitosis is the only mechanism that allows asexual creatures to survive.Learn more:
https://brainly.com/question/3327479?referrer=searchResults

An experiment was set up as diagrammed below to measure the amount of O2 (red) and CO2 (blue) over time using live Spinach leaves and sensor probes for these gases. The results from this experiment are graphed for you. SARRON GOOD GNYGA fo a Figure 1 0.8- 06 04- 10 15 Time (min) 205 204 203 202 201 0 10 15 Time (min) (a) State which metabolic process occurred in this apparatus. (b) Explain the graphed results related to that process.
Answers
The metabolic process that occurred in the apparatus of the experiment given in the question is photosynthesis. The graphed results of the experiment are related to the process of photosynthesis.
Photosynthesis is the process in which the green plants use the energy of sunlight to convert carbon dioxide and water into glucose and oxygen. In this process, chlorophyll pigment, present in the chloroplasts of the plant cells, captures the energy of sunlight. This captured light energy is used to convert water and carbon dioxide into oxygen and glucose.
In the experiment mentioned above, the metabolic process that occurred in the apparatus is photosynthesis. It is because spinach leaves were used in the experiment to observe the amount of oxygen (O2) and carbon dioxide (CO2) present in the leaves.
The graphed results of this experiment show that the amount of oxygen in the leaves increased over time while the amount of carbon dioxide decreased. This is because the leaves absorbed carbon dioxide from the air and converted it into glucose and oxygen through the process of photosynthesis. As a result, the amount of oxygen increased over time, and the amount of carbon dioxide decreased. Hence, it can be concluded that the graphed results of the experiment are related to the process of photosynthesis.
More on photosynthesis: https://brainly.com/question/29764662
#SPJ11