Is carbon a metal or a nonmetal? How many valence electrons does a carbon atom have?
Answers
Answer:
Carbon is a solid non-metal element. Carbon has eight valence electrons.
Explanation:
Hope this helps!!
Related Questions
What element has 2 valence electrons and 2 energy levels
Answers
Answer:
helium
Explanation:
helium has 2 protons and 2 electrons. The 2 electrons are on the first energy level.
a restriction enzyme recognizes the sequence 5ʹ-gtcatgac-3ʹ and makes staggered cuts. which statement is most likely to be true?
Answers
The most likely true statement about a restriction enzyme recognizing the sequence 5ʹ-gtcatgac-3ʹ and making staggered cuts is: "The restriction enzyme produces fragments with sticky ends".
When a restriction enzyme recognizes a specific DNA sequence, it cuts the DNA at or near that sequence. Staggered cuts refer to cuts made at different positions on the two DNA strands, resulting in fragments with overhanging ends. These overhanging ends are often referred to as sticky ends because they can base pair with complementary sequences.
In the given sequence 5ʹ-gtcatgac-3ʹ, the restriction enzyme would recognize and cut between the G and the T bases, resulting in staggered cuts. This would produce fragments with complementary overhangs: 5ʹ-GTCATG-3ʹ and 5ʹ-GTCA-3ʹ. These overhanging ends can then bind or anneal with complementary sequences during DNA manipulation, such as in cloning or DNA ligation reactions.
Learn more about restriction enzymes at https://brainly.com/question/15278286
#SPJ11
Which of the following is not a characteristic of a solution?What is the concentration of a solution?
Answers
Answer: B. it will scatter a beam of light
Explanation: hope it is helpful........
what is the character of the vapor after each condensation-vaporization cycle in a fractional distillation of a mixture?
Answers
How many moles is 1.5 x10 20?
Answers
2.49 x \(10^{-4}\) moles is equal to 1.5×\(10^{20}\). The number of moles is equal to the number of molecules divided by Avogadro's number, 6.022×\(10^{23}\).
This means that 1.5×\(10^{20}\)moles are equal to
1.5×\(10^{20}\)/6.022×\(10^{23}\)
0.24908×\(10^{-3}\)
2.49 x \(10^{-4}\) moles.
Avogadro's number: Avogadro's number is a constant used in chemistry to represent the number of atoms or molecules in a mole of a substance. It is equal to 6.022 x \(10^{23}\). Or we can say that Avogadro's number is, the number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 1023. The units may be electrons, atoms, ions, or molecules, depending on the nature of the substance and the character of the reaction.
Learn more about moles:
https://brainly.com/question/28037816
#SPJ4
How many molecules are in 32 grams of NH3?
Answers
Answer:
1.87897962011728
Explanation:
Is argon made of atoms or molecules?
Answers
ELEMENTS ARE ATOMS
brainliest?
3. Which of the following is NOT a major ingredient in weather?
A clouds
B sunshine
Cwater
D air
Answers
Answer:
I would say sunshine
Explanation:
the major ingredients are...
The four basic ingredients of weather and climate are pressure, moisture, wind, and temperature.
hope this helps!!
and please give me brainliest! :)
The option that is not a major ingredient in weather is B. Sunlight.
Weather simply means the state of the atmosphere at a particular place and time. It should be noted that the weather of a place can be different from another place. For example, a place can be sunny while another can be rainy.
The atmospheric phenomena that are the ingredients of weather are clouds, water, and air. Based on the question asked, sunlight isn't an ingredient.
Read related link on:
https://brainly.com/question/20406044
Calculate the pH of a solution containing 0.085 M nitrous acid(HNO2; Ka = 4.5 x 10-4) and 0.10 potassium nitrite (KNO2).
Answers
The pH of a solution containing 0.085 M nitrous acid (HNO2; Ka = 4.5 x 10-4) and 0.10 M potassium nitrite (KNO2) can be calculated using the principles of acid-base equilibrium.
1. The solution will be slightly acidic, and the pH value can be determined by the concentration of H+ ions resulting from the ionization of nitrous acid.
2. The pH of the solution can be calculated by considering the ionization of nitrous acid and the hydrolysis of the nitrite ion. Nitrous acid (HNO2) partially ionizes in water to form hydronium ions (H3O+) and nitrite ions (NO2-). This ionization can be described by the equation: HNO2 ⇌ H+ + NO2-.
3. The equilibrium constant for this reaction is given by the acid dissociation constant (Ka) for nitrous acid, which is 4.5 x 10-4. Since the concentration of HNO2 is 0.085 M, we can assume that x moles of HNO2 ionize, resulting in x moles of H+ ions and x moles of NO2- ions. Therefore, the concentration of H+ ions can be approximated as x M.
4. The nitrite ions (NO2-) from the potassium nitrite (KNO2) can undergo hydrolysis in water to produce hydroxide ions (OH-) according to the reaction: NO2- + H2O ⇌ HNO2 + OH-
5. Since the concentration of KNO2 is 0.10 M, we can assume that x moles of NO2- ions hydrolyze, resulting in x moles of HNO2 and x moles of OH- ions. Therefore, the concentration of OH- ions can be approximated as x M.
6. To determine the pH, we need to calculate the concentration of H+ ions in the solution. Since the reaction of nitrous acid and the hydrolysis of nitrite ions occur simultaneously, we need to consider their combined effect on the concentration of H+ ions. The net effect will depend on the relative magnitudes of the ionization constant (Ka) and the hydrolysis constant (Kw).
7. In this case, the concentration of nitrous acid (0.085 M) is much greater than the concentration of nitrite ions (0.10 M), indicating that the ionization of nitrous acid is dominant. Therefore, the concentration of H+ ions can be approximated as x M.
8. To calculate x, we can use the expression for the acid dissociation constant (Ka) of nitrous acid: Ka = [H+][NO2-] / [HNO2]
Substituting the known values, we get:
4.5 x 10-4 = x * x / (0.085 - x)
9. Solving this equation will yield the value of x, which represents the concentration of H+ ions. From there, we can calculate the pH using the formula pH = -log[H+].
10. In summary, the pH of the solution can be calculated by considering the ionization of nitrous acid (HNO2) and the hydrolysis of nitrite ions (NO2-). The equilibrium between these reactions will determine the concentration of H+ ions, which in turn determines the pH value. The concentration of H+ ions can be approximated by assuming that the dominant reaction is the ionization of nitrous acid due to its higher concentration compared to nitrite ions. By solving the relevant equations, the concentration of H+ ions can be determined, and the pH of the solution can be calculated using the formula pH = -log[H+].
Learn more about hydronium ions here: brainly.com/question/13387755
#SPJ11
draw the curved-arrow mechanism for the reaction of o-vanillin with p-toluidine to produce the expected imine, 2-methoxy-6-(p-tolyliminomethyl)phenol, via nucleophilic addition-elimination
Answers
The reaction of o-vanillin with p-toluidine to produce the expected imine, 2-methoxy-6-(p-tolyliminomethyl)phenol, involves nucleophilic addition-elimination.
The curved-arrow mechanism includes the attack of the amine nitrogen on the aldehyde carbon, followed by proton transfer and elimination of water. The lone pair of electrons on the amine nitrogen attacks the electrophilic carbon of the aldehyde, forming a new bond and displacing the pi bond to oxygen. The resulting intermediate undergoes a proton transfer, where a proton from the nitrogen is transferred to the oxygen atom. The elimination of a water molecule occurs, forming the imine and regenerating the catalyst.The curved-arrow mechanism illustrates the movement of electrons and the bond changes during the reaction, providing a visual representation of the reaction steps.
learn more about reaction here:
https://brainly.com/question/30464598
#SPJ11
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2V2 V1/T1 = V2/T2 PV = nRT V1/T1 x V2/T2

Answers
Answer:
Option b (V1/T1 = V2/T2) is the right alternative or the new volume will be "0.048 L".
Explanation:
The given values are:
Temperature,
T₁ = 25°C
or,
= 298.15 K
T₂ = 50°C
or,
= 323.15 K
Volume,
V₁ = 45 mL
or,
= 0.045 L
V₂ = ?
As we know,
⇒ \(\frac{V_1}{T_1} =\frac{V_2}{T_2}\)
Or,
⇒ \(V_2=\frac{V_1\times T_2}{T_1}\)
On substituting the values, we get
⇒ \(=\frac{0.045\times 323.15}{298.15}\)
⇒ \(=\frac{14.541}{298.15}\)
⇒ \(=0.048 \ L\)
I need help :(( picture included

Answers
The given reaction is photosynthesis. The Six molecules of carbon dioxide are involved in this reaction.
What is photosynthesis?Photosynthesis is the process of the formation of sugar from carbon dioxide and water.
1 molecule of sugar is produced in the reaction.
The Six molecules of carbon dioxide are involved in this reaction.
There are six molecules of carbon on the left-hand side of the equation.
There are six molecules of carbon on the right-hand side of the equation.
There are Twelve molecules of hydrogen on the left-hand side.
There are 12 molecules of hydrogen on the right-hand side.
There are Eighteen oxygen molecules on the left hand side.
There are eighteen oxygen molecules on the right hand side.
Therefore, The given reaction is photosynthesis. The Six molecules of carbon dioxide are involved in this reaction.
To learn more about photosynthesis, refer to the link:
https://brainly.com/question/1388366
#SPJ1
At a given temperature, 0.500 mols of CO and 1.50 moles of water vapor are added to a 2.50 L vessel. When the reaction reaches equilibrium, the [CO2] and [H2] are 0.00775 M. Find the [CO] and the [H2O] at equilibrium. Calculate the Keq and predict the sign of ΔG.
Answers
The concentrations of the reaction's reactants and products must be equal at equilibrium. Following is a description of how CO and H2O react to generate CO2 and H2: CO + H2O <=> CO2 + H2 We can determine the equilibrium CO and H2O concentrations using the available data.
The starting concentrations of CO and H2O are 0.800 M and 0.800 M, respectively, due to the total moles of CO and H2O being 2.00 moles and the total volume being 2.50 L. The equilibrium expression may be used to compute the equilibrium concentrations of CO and H2O: K = [CO2][H2]/[CO][H2O] K = (0.00775)(0.00775)/[CO] may be used to derive the equilibrium constant given that [CO2] and [H2] are both equal to 0.00775 M.
[H2O] K = (0.00775)(0.00775)/[0.0455], when the equilibrium concentrations of CO and H2O are plugged in.[0.0455]. ][0.0455] K = 0.0020 From this, we can calculate the equilibrium concentrations of CO and H2O: [CO] = 0.0455 M [H2O] = 0.0455 M .
The standard free energy change (G°), which can be calculated using the formula G° = -RTlnK, may be used to estimate the sign of G for this reaction. Since K > 1, we may anticipate a spontaneous response, meaning that G will be negative.
Learn more about concentrations at:
https://brainly.com/question/10725862
#SPJ1
Which of the following statements about pericyclic reactions is true? Multiple Choice In pericyclic reactions, bonds are broken and formed in multiple steps. In pericyclic reactions, all bonds are broken and formed in a single step. One Intermediate has been identified in pericyclic reactions. The transition state in a pericyclic reaction is acyclic.
Answers
The correct statement about pericyclic reactions is:
In pericyclic reactions, all bonds are broken and formed in a single step.
Pericyclic reactions are a class of organic reactions characterized by the simultaneous breaking and forming of multiple bonds in a concerted manner, meaning that all bond changes occur in a single step. This is in contrast to stepwise reactions where bonds are broken and formed in separate steps.
In a pericyclic reaction, the reactants rearrange their electrons in a concerted fashion, leading to the formation of new bonds and the breaking of existing bonds. This concerted process occurs through the overlap of molecular orbitals, allowing for the efficient transfer of electrons.
Pericyclic reactions are concerted reactions in which the breaking and forming of bonds occur simultaneously in a single step. They do not involve multiple steps or intermediates. The transition state in a pericyclic reaction is cyclic, not acyclic.
Learn more about pericyclic reactions from the link given below.
https://brainly.com/question/33440345
#SPJ4
why is it a problem of habitat destruction?
Answers
Answer:
because he must be not healthy person
Answer:
people find that 5hey are self sabotaging them selves to move forward
How many moles are present in 5.24 x 1023 molecules of CH4?
Answers
Answer:
0.807 moles
Explanation:
No of molecules = No of mole × Avogadro's number
No of mole = No of molecules / Avogadro's number
No of mole = 5.24×10^23/6.02×10^23
0.870 moles
HELP ASAP PLSSS 3 AND 4
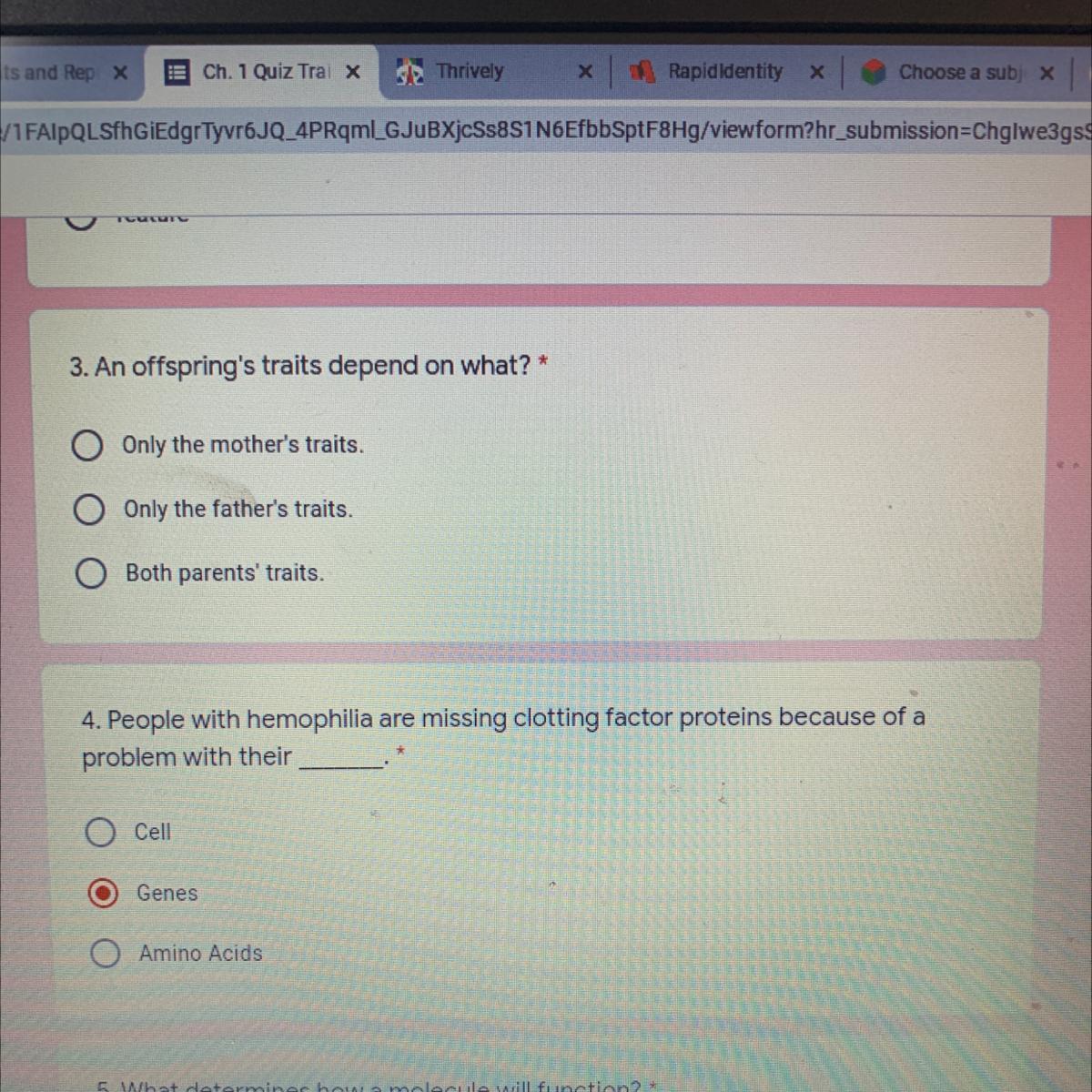
Answers
Answer:
both parents traits and genes
Quantization of energy lab Hypothesis: Make a prediction that describes the relationship between the composition of an unknown substance and its emission spectrum. For example, “If each element can be identified by its ______________, then the ____________ of an unknown star can be determined." variables: independent - dependent- control-
Answers
Answer:
If each element can be identified by its spectrum then the composition of an unknown star can be determined
Explanation:
The chemical nature of the elements is that they absorb specific wavelength of light depending on their atom. By spectral analysis of the spectrum of emitted light by a body, the body's composition can therefore be determined. As such in order to determine the composition of distant bodies such as planets, stars and other celestial bodies scientists usually make use of spectroscopy.
Answer:
If each element can be identified by its spectrum then the composition of an unknown star can be determined
Explanation:
How Do You Get Different Coloured Fireworks? ...
Answers
Determine the molecular formula of a compound whose molecular mass is 60. 00 g/mol and has an empirical formula of ch4n.
Answers
The molecular formula of a compound is C2H8N2 that is determined by empirical formula.
Molecular formula of a compound whose molecular mass is 60.00 g/mol and has an empirical formula of C2H8N2. We know the empirical formula and thus the molar mass of the empirical formula, we simply need to find out how many of these fit into the molar mass of the molecular formula.
In this problem, we have an empirical formula of CH4N
So the molar mass is 12 + 4 + 14 = 30 g/mol.
Molecular formula mass/Empirical formula mass=60 g/mol/30 g/mol=2
The molecular formula is twice that of the empirical formula.
Molecular formula = 2XCH4N = C2H8N2
To learn more molecular formula check the link below:
https://brainly.com/question/26388921
#SPJ4
Why is creativity important in scientific investigations?
a. Creative solutions are often needed to solve technical problems.
b. Scientists need to creatively adjust their data to prove their hypothesis.
c. There is no way to test a hypothesis, so creativity is necessary.
d. Most scientific experiments have no data.
Answers
Answer:
a. Creative solutions are often needed to solve technical problems.
Explanation:
Science in itself is a voyage of discovery. Scientific investigators focusing on various issues are often working hard to find creative solutions to complex technical problems.
In order to find solutions to all these problems bedeviling humanity, scientific investigators must be quite creative. Creativity is always at the bedrock of finding solutions to the complex technical problems of the world which happens to be the ultimate goal of science.
Hence, creativity is indispensable in scientific investigation and creative solutions are often needed to solve technical problems.
Answer:
a. Creative solutions are often needed to solve technical problems
Explanation:
Scientific investigation is the process using the scientific method which consists of empirical techniques in obtaining answers to question raised and used for driving the investigation
The scientist carrying out the investigation into the new area, is therefore required to be creative in adapting the available resources in answering the questions based on the scientific method
Help me with this. I dont even know what this compound is...

Answers
Answer:
c
Explanation:
Answer
the other person is right its c
Explanation:
none
calculate+the+empirical+formula+from+the+given+percent+compositions.+82%+nitrogen+(n),+18%+hydrogen+(h)
Answers
The mole ratio for 82% nitrogen (N) and 18% hydrogen (H) is roughly 1:3. As a result, the compound's empirical formula is NH₃ (one nitrogen and three hydrogen atoms).
To calculate the empirical formula from the given percent compositions, we need to convert the percentages into moles and find the simplest whole-number ratio between the elements. Here's the calculation:
Assuming we have 100 grams of the compound, we would have:
- 82 grams of nitrogen (N)
- 18 grams of hydrogen (H)
Now, we need to convert these masses into moles using the molar mass of each element:
- Nitrogen (N): 1 mole of N = 14.01 grams
\(\begin{equation}\text{Moles of N} = \frac{82 \text{ grams}}{14.01 \text{ g/mol}} \approx 5.85 \text{ mol}\)
- Hydrogen (H): 1 mole of H = 1.01 grams
\(\[\text{Moles of H} = \frac{18 \text{ g}}{1.01 \text{ g/mol}} \approx 17.82 \text{ mol}\]\)
Next, we need to find the simplest whole-number ratio between nitrogen and hydrogen by dividing each number of moles by the smaller value (5.85 mol, in this case):
\(\[\text{Moles of N (rounded)} = \frac{5.85 \text{ mol}}{5.85 \text{ mol}} = 1\]\)
\(\[\text{Moles of H (rounded)} = \frac{17.82 \text{ mol}}{5.85 \text{ mol}} \approx 3.04\]\)
The ratio between N and H is approximately 1:3, so the empirical formula of the compound is NH₃ (1 nitrogen atom, 3 hydrogen atoms).
To know more about the mole ratio refer here :
https://brainly.com/question/32125056#
#SPJ11
you decide to test your pillbugs' preference for an acidic environment versus a nonacidic environment. on one side of the chamber you place filter paper moistened with water. what is appropriate to place on the other side to test this variable?
Answers
you decide to test your pillbugs' preference for an acidic environment versus a nonacidic environment. on one side of the chamber you place filter paper moistened with water. Dry filter paper is appropriate to place on the other side to test this variable.
ABOUT PILLBUGSArmadillidiidae (Pillbugs) is a family of woodlice, a terrestrial crustacean group in the order Isopoda. Unlike members of some other woodlice families, members of this family can roll into a ball, an ability they share with the outwardly similar but unrelated pill millipedes and other animals. This ability gives woodlice in this family their common names of pill bugs or roly polies.Other common names include slaters, potato bugs, and doodle bugs. Most species are native to the Mediterranean Basin, while a few species have wider European distributions. The best-known species, Armadillidium vulgare, was introduced to New England in the early 19th century and has become widespread throughout North America.
Learn more about pillbugs at
https://brainly.com/question/26410019.
#SPJ4
How many half-lives are required for the concentration of reactant to decrease to 12. 5% of its original value?.
Answers
Answer:
Three half-lives
Explanation:
12.5% is one-eighth. One eighth is the cube of one half, so it would take three half-lives to reduce a reactant's concentration to 12.5%
Three half-lives are required for the concentration of reactant to decrease to 12. 5% of its original value.
What is half life?Half -life of a substance is defined as the time which is required for half of the quantity of a radioactive substance to get decayed.It is a term which is used in nuclear chemistry for describing how quickly unstable atoms undergo radioactive decay into other nuclear species by emitting particles or the time which is required for number of disintegrations per second of radioactive material to decrease by one half of its initial value.
In the given example 12.5 % is 1/8 and three times 1/2 that is 1/2×1/2×1/2=1/8 , hence three half lives are required for the concentration of reactant to decrease to 12. 5% of its original value.
Learn more about half-life,here:
https://brainly.com/question/24710827
#SPJ2
Which of the following is true of the relationships between heat of fusion and heat of vaporization?
A.) Using the heat of fusion, you can predict the heat of vaporization.
B.) The heat of vaporization is typically larger than the heat of fusion.
Answers
Answer:
The heat of vaporization is typically larger than the heat of fusion
Next question answer:
The liquid water absorbs heat from the skin surface and is transferred to the air when the water evaporates.
Explanation:
Answer:
First answer
B. The heat of vaporization is typically larger than the heat of fusion
Second answer:
A. The liquid water absorbs heat from the skin surface and is transferred to the air when the water evaporates.
Explanation:
Examine the diagram shown. Which are the correct labels for continental plates 1 and 2?
2
equator
1
1 = Antarctic, 2 = African
1 = Indo-Australian, 2 = Eurasian
1 = North American, 2 = Pacific
1 = South American, 2 = Antarctic
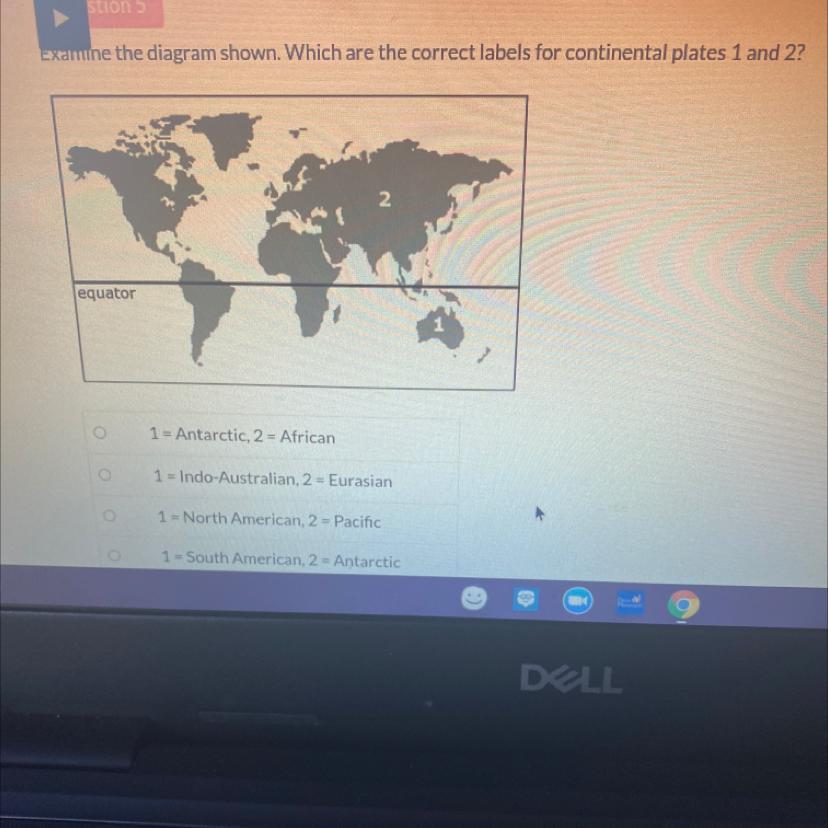
Answers
Answer:
Australian and eurasian
Explanation:
What is the necessary voltage to power the electrolysis of molten sodium chloride?
Answers
The necessary voltage to power the electrolysis of molten sodium chloride is 4V.
Electrolysis is the breakdown of a compound into the elements it is formed from by passing electricity through it. It is actually a chemical reaction in which one element loses electrons and the other may gain electrons. The process of electrolysis has wide applications in electroplating, metallurgical processes, production of chlorine gas, etc.
Molten sodium chloride is generally used in the production of chlorine gas and sodium metal. This molten or aqueous form of sodium chloride is also known as brine. The process is carried out in a cell called the Down's cell.
To know more about electrolysis, here
brainly.com/question/12054569
#SPJ4
Which choice confirms that one air mass is being replaced by another air mass in a given area?
A. increase in wind
B. high air pressure
C. high air temperature
D. it is impossible to tell.
Answers
Answer:
Answer is A
Explanation:
Increase in wind
if sneezing is the response to smelling food is it conditioned or unconditioned
Answers
Answer:
i think its unconditioned response.hope it helpsstay safe healthy and happy.