Answers
Answer:
The diffrence between Fe2+ and Fe3+ is the Fe2+has a pale green color and turns violet when water is added to it. While Fe3+ forms blood red when it reacts with thiocyanate ions.
Fe2+ has paramagnetic properties whereas
Fe3+ has diamagnetic properties.
Explanation:
I may not be correct.
Answer:
ok ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,
Related Questions
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
When the volume of a gas is changed from 11.5 cm3 to __ cm3, the temperature will change from 415.0 K to 200.0 K

Answers
Answer:
Explanation:
Charles Law: The volume of and ideal gas is directly proportional to the temperature when the pressure is kept constant
V₁ - initial Volume = 11.5 cm³ ; T₁ = Initial temperature = 415 K ;
V₂ - final volume ;T₂ =final temperature = 200 K
\(\dfrac{V_{1}}{T_{1}}=\dfrac{V_{2}}{T_{2}}\\\\\\\dfrac{11.5}{415}=\dfrac{V_{2}}{200}\\\\\\\dfrac{11.5}{415}*200=V_{2}\\\\\\V_{2}=5.5 \ cm^{3}\)
For the following reaction, 4.77 grams of carbon (graphite) are allowed to react with 16.4 grams of oxygen gas.
carbon (graphite) (s) + oxygen (g) → carbon dioxide (g)
1. What is the maximum amount of carbon dioxide that can be formed?
2. What is the FORMULA for the limiting reagent?
3. What mass of the excess reagent remains after the reaction is complete?
Answers
Answer:
1. 17.5 g of CO₂
2. The limiting reactant is carbon (graphite), and its formula is C(graphite)
3. 3.7 g of O₂
Explanation:
First, we have to write the chemical equation for the reaction. For this, we have to know the chemical formula of each reactant and product:
Reactants: carbon(graphite) ⇒ C(graphite) ; oxygen gas ⇒ O₂(g)Products: carbon dioxide ⇒ CO₂(g)Thus, we write the chemical equation:
C(graphite) + O₂(g) → CO₂(g)
The equation is already balanced because it has the same number of C and O atoms on both sides. Thus, we can see that 1 mol of C(graphite) reacts with 1 mol of O₂ and produce 1 mol of CO₂ (mole-to-mole reaction).
Now we convert the grams of reactants to moles by using the molecular weight (Mw) of each compound:
Mw(C) = 12 g/mol
moles of C(graphite) = 4.77g/(12 g/mol) = 0.3975 mol
Mw(O₂) = 16 g/mol x 2 = 32 g/mol
moles of O₂ = 16.4 g/(32 g/mol) = 0.5125 mol
Now, we can compare the stoichiometric ratio (given by the moles of reactants in the equation) with the actual ratio (given by the mass of reactants we have):
stoichiometric ratio ⇒ 1 mol C(graphite)/mol O₂
actual ratio ⇒ 0.3975 mol C(graphite)/0.5125 mol O₂
We can see that we need 0.3975 moles of O₂ to react with C(graphite) and we have more moles (0.5125 mol) so the excess reactant is O₂. Thus, the limiting reactant is C(graphite).
The amount of product (CO₂) that is formed is calculated from the amount of limiting reactant. We can see in the chemical equation that 1 mol of CO₂ is produced from 1 mol of C(graphite) ⇒ stoichiometric ratio = 1 mol CO₂/mol C(graphite).
Thus, we multiply the moles of C(graphite) we have by the stoichiometric ratio to calculate the moles of CO₂ produced:
moles of CO₂ = 0.3975 mol C(graphite) x 1 mol CO₂/mol C(graphite) = 0.3975 mol CO₂
Now, we convert the moles of CO₂ to mass by using the Mw:
Mw(CO₂) = 12 g/mol + (16 g/mol x 2) = 44 g/mol
mass of CO₂ = 0.3975 mol CO₂ x 44 g/mol CO₂ = 17.5 g
Therefore, the maximum amount of carbon dioxide (CO₂) formed is 17.5 g.
Since this is a mole-to-mole reaction, the moles of excess reactant that remains after the reaction is complete is calculated as the difference between the moles of excess reactant and limiting reactant:
remaining moles of O₂ = 0.5125 mol - 0.3975 mol = 0.115 mol O₂
Finally, we convert the moles of O₂ to mass with the Mw (32 g/mol) :
mass of O₂ = 0.115 mol O₂ x 32 g/mol = 3.68 g
Therefore, the mass of the excess reagent that remains after the reaction is complete is 3.7 g.
A 3 column table with 7 rows. The first column is labeled color with entries red, orange, yellow to white, white, white to blue, blue, blue. The second column is labeled surface temperature in Kelvin with entries under 3500, 3500 to 5000, 5000 to 6000, 6000 to 7500, 7500 to 11000, 11000 to 25000, greater than 25000. The last column is labeled average mass in solar masses with entries, 0.3, 0.8, 1.1, 1.7, 3.2, 18, 60.
Arcturus is a star whose surface temperature is 4290 K.
What is most likely the color of Arcturus?
What is most likely the mass of Arcturus?
about
solar masses
Answers
From the table as we can see it ;
The color of the star is orangeThe average mass of the star is 0.8What is a table?A table is often used for the representation of information. We know that a table has to have columns. Each of the columns could be used to show particular variable. Now we have the temperature, the color and the mass of different stars showed on the table as we can see it in the question.
We now have the assignment to find the color and mass of the start that corresponds to a temperature of about 4290 K hence we have to look intently at the table.
Color of the star - Orange
Average Mass of the star - 0.8
Learn more about tabular presentation:https://brainly.com/question/15883059?
#SPJ1
Answer: What is most likely the color of Arcturus? ORANGE
What is most likely the mass of Arcturus? about 0.8 solar masses
Explanation: just did the assignment
How many grams of NaOH are needed to make 100. mL of solution with a concentration of 1.5 M?
Answers
To create 100 mL of solution with a concentration of 1.5 M, 6.00 grams of NaOH are required.
The amount of NaOH needed to make 100. mL of solution with a concentration of 1.5 M can be calculated using the formula:
mass = molarity x volume x molar mass
where:
molarity = 1.5 M (given)
volume = 100. mL = 0.1 L (given)
molar mass of NaOH = 40.00 g/mol (from periodic table)
Substituting the values, we get:
mass = 1.5 mol/L x 0.1 L x 40.00 g/mol
mass = 6.00 g
Therefore, 6.00 grams of NaOH are needed to make 100. mL of solution with a concentration of 1.5 M.
To know more about the Solution, here
https://brainly.com/question/14296204
#SPJ1
HELP WHAT NUMBER IS THIS??
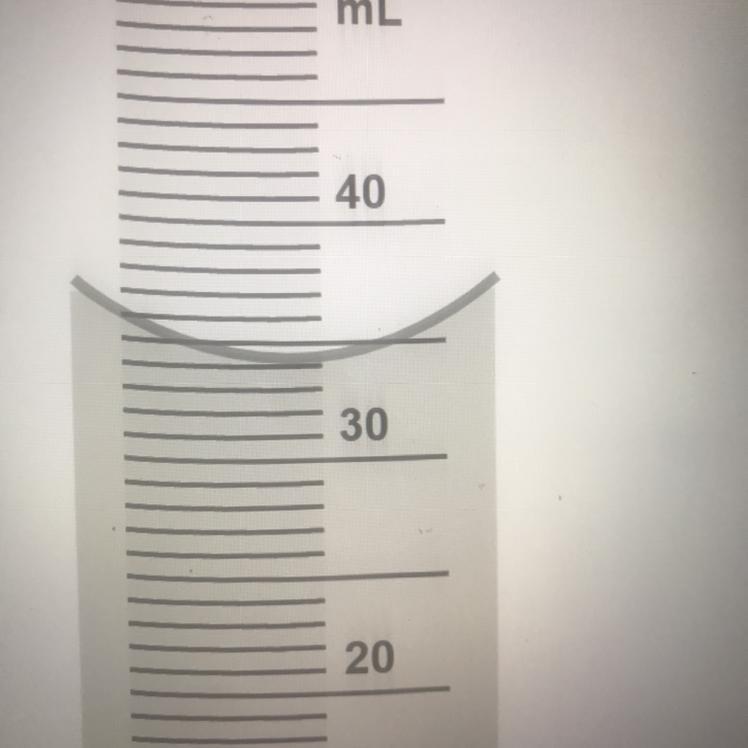
Answers
which 2 elements are similar?

Answers
Answer: the elements that are the most similar are in the same group, or column of the periodic table
Explanation:They have similar chemical properties
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
what happens to a rock that has rainwater entering its cracks and then freezing
Answers
Answer:
Physical weathering is caused by the effects of changing temperature on rocks, causing the rock to break apart.
Explanation:
Freeze-thaw occurs when water continually seeps into cracks, freezes and expands, eventually breaking the rock apart.
Consider the reaction below:
2 CO(g) + O₂(g) ⇌ 2 CO₂(g)
If Kc is 2.24 × 10²² at 1273.0 °C, calculate Kp at the same temperature.
Answers
The Kc is 2.8 * 10^24
What is the Kp?In chemistry, Kp usually refers to the equilibrium constant of a reaction that involves gases. It is defined as the ratio of the partial pressures of the products to the partial pressures of the reactants, each raised to the power of their stoichiometric coefficients.
Kp= Kc (RT)^Δn
Thus;
Kc = Kp/(RT)^Δn
Kc = 2.24 × 10²² /(0.082 * 1546)^-1
Kc = 2.8 * 10^24
Thus the Kc of the reaction when we consider the concentration of the reactants is 2.8 * 10^24
Learn more about Kp:https://brainly.com/question/22074421
#SPJ1
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
What would change oxygen into another isotope
Answers
Answer:
Adding Neutrons
General Formulas and Concepts:
Chemistry - Atomic Structure
Atoms CompositionNucleus - Protons, NeutronsElectronsExplanation:
We know that adding Protons to any atom will change its chemical properties and make it a different element.
We also know that adding Electrons to any atom will simply only change its overall charge.
Isotopes are formed by adding neutrons to the nucleus. It will be the same element but it would have a different mass and amount of neutrons.
PLS HELP ME WITH MY CHEMISTRY

Answers
Answer:
\( \boxed{8.9 \: g /cm^3}\)
Explanation:
Given:
Mass of sample= 39.2 g
Volume occupied= 4.4 cm³
To find:
Density=?
Solution:
Formula to be used,
\(\sf Density = \frac{mass}{volume} \)
\(\sf Density = \cancel{ \frac{39.2}{4.4} } = 8.9 \bar{0} \bar{9} \: g /cm^3\)
The rounder figure answer upto tenths place can be written as 8.9 g/cm³
\(\sf \small \pink{Thanks }\: \green{for} \: \blue{joining} \: \orange{brainly } \: \red{community}!\)
Which of the following generally determines if a reaction will occur?
A. pH
B. Equilibrium constant
O C. Kinetics
D. Thermodynamics
Answers
Answer:
D - Thermodynamics
Explanation: I just took the quiz
Bohr's Model of the atom
If an element has 7 electrons in its valence shell (outermost ring), which chemical family would you expect it to belong to?
Answers
Answer:
group 17 the halogen.as it has 7 electron in its outermost ring
2NO(g) + O₂(g) = 2NO₂(g)
AH = -112 kJ K = 0.50
The equilibrium concentrations are
[NO] = 0.31 M, [02] = 1.10 M, and
[NO2] = [?]
What is the equilibrium concentration of
NO2 at this temperature?
![2NO(g) + O(g) = 2NO(g)AH = -112 kJ K = 0.50The equilibrium concentrations are[NO] = 0.31 M, [02] = 1.10](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/YRGcrEmw6OtcUiE6tB5KUryscULOSlX5.png)
Answers
The equilibrium concentration of NO₂ at this temperature is approximately 0.219 M.
To determine the equilibrium concentration of NO₂, we can use the equilibrium constant expression (Kc) and the given equilibrium concentrations of NO and O₂. The equilibrium constant expression for the given reaction is:
Kc = ([NO₂]²) / ([NO]²[O₂])
We are given the equilibrium concentrations of NO and O₂ as [NO] = 0.31 M and [O₂] = 1.10 M, respectively. We need to find the equilibrium concentration of NO₂, denoted as [NO₂].
Using the given equilibrium concentrations and the equilibrium constant expression, we can rearrange the equation and solve for [NO₂]:
Kc = ([NO₂]²) / ([NO]²[O₂])
0.50 = ([NO₂]²) / ((0.31 M)²(1.10 M))
0.50 = ([NO₂]²) / (0.0961 M³)
Multiplying both sides by 0.0961 M³, we have:
0.04805 M³ = [NO₂]²
Taking the square root of both sides, we find:
[NO₂] = √(0.04805 M³)
[NO₂] ≈ 0.219 M
Therefore, the equilibrium concentration of NO₂ at this temperature is approximately 0.219 M.
It's important to note that the units of concentration (M) were used throughout the calculations, and the answer is rounded to three significant figures based on the given data.
Additionally, the negative sign of the enthalpy change (AH) indicates an exothermic reaction, and the equilibrium constant (K) of 0.50 suggests that the reaction favors the products, as the concentration of NO₂ is greater at equilibrium.
For more such questions on equilibrium concentration visit:
https://brainly.com/question/13414142
#SPJ8
I am so confused about this. Please some chemistry genius helps me.
Thank you in advance, you are special!

Answers
use law of conservation mass .
In a chemical reaction mass is neither created nor destroyed.
Hence Mass of reactants=Mass of products.
Al=7.5gCl_2=24.4gMass of Product:-
\(\\ \sf\longmapsto 7.5+24.4\)
\(\\ \sf\longmapsto 31.9g\)
Which one is not an organic coumpounds

Answers
Answer:
A
Explanation:
There are many definitions, and all of them are organic under some definition. The answer is probably A because it is the only one without hydrogen, and sometimes molecules without hydrogen are counted as being inorganic. A is CCl2F2, which is comprised of two chlorine atoms, two fluorine atoms, and a carbon atom.
Describe how crystals change if they form in large amounts of space vs limited space.
Answers
Crystals form when magma cools because the solution is super-saturated with certain minerals. Because the crystals do not have much time to form if the magma cools quickly, they are very small. The crystals have enough time to grow and become large if the magma cools slowly.
What causes big crystals to form?When the solution becomes supersaturated, which means there is too much salt dissolved in the water, crystals form. Crystals form from the extra salt (or other material). To obtain a supersaturated solution, either cool the solution or allow some of the water to evaporate.
The size of the crystals is affected by the solubility of the compound in the solvent used for recrystallization, the number of nucleation sites, mechanical agitation of the system, and time during crystal growth.
Thus, the crystals do not have much time to form if the magma cools quickly, they are very small.
To learn more about the crystals, follow the link;
https://brainly.com/question/13008800
#SPJ9
the average human lives 74 years. how many seconds is this? write your answer in scientific notation
Answers
Number of second in human lives in scientific notation is 3.9 × 10⁷ second
Given that;
Average human lives = 74 years
Find:
Number of second in human lives in scientific notation
Computation:
Number of second in human lives in scientific notation = 74 × 365 × 24 × 60
Number of second in human lives in scientific notation = 38,894,400
Number of second in human lives in scientific notation = 3.9 × 10⁷ second
Learn more:
https://brainly.com/question/3712546?referrer=searchResults
if scientists found a fossil in the middle layer that was 2 million years old, would they think Earth was more or less than 2 million years
Answers
The discovery of a 2-million-year-old fossil would align with the existing understanding of Earth's geological history, as it falls within the timeframe of the Quaternary period.
If scientists found a fossil in the middle layer that was determined to be 2 million years old, they would not automatically conclude that Earth was younger or older than 2 million years. The age of the fossil would provide valuable information about the minimum age of the layer in which it was found, but it would not necessarily provide conclusive evidence about the age of the entire Earth.
To determine the age of Earth, scientists rely on a variety of dating methods, including radiometric dating of rocks and minerals, as well as the study of isotopes and geological processes. These methods provide estimates of Earth's age in the billions of years.However, it would not significantly alter the prevailing scientific consensus that Earth is approximately 4.5 billion years old. The age of Earth is based on a comprehensive body of evidence from multiple disciplines and dating techniques, and a single fossil would not overturn that understanding.
for such more questions on period
https://brainly.com/question/29752383
#SPJ8
Choose the correct statement. At a higher temperature……………..
1.Energies of particles increase
2.Rate of reaction increases
3.Particles collide more frequently
4.All correct
Answers
Answer:
4. All correct
Mark me brainliest and thenks :)))
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0610 M , [Fe3+]=0.0389 M , [Sn4+]=0.00744 M , and [Fe2+]=0.01196 M . Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
Answers
The cell potential of the system is obtained as 0.66 V.
What is the cell potential?We know that we can be able to make use the table of the standard potentials so as to be able to obtain the cell potentials under standard conditions.
We have that;
The standard cell potential can be obtained as;
Cell potential of the cathode - Cell potential of the anode
0.77 V - 0.15 V
= 0.62 V
By the Nernst equation;
E = E° - 0.0592/n log Q
E = cell potential under the given conditions
n = Number of electrons transferred
E° = standard cell potential
Q = reaction quotient
Then;
Q = [0.00744] [0.01196]/[0.0610] [0.0389]
= 8.9 * 10^-5/2.4 * 10^-3
= 3.7 * 10^-2
Thus;
E = 0.62 - 0.0592/2 log (3.7 * 10^-2)
E = 0.66 V
Learn more about Nernst equation:https://brainly.com/question/13043546
#SPJ1
How many mL are in 0.365 L?
Answers
Answer:
365 mL
Explanation:
There are 1,000 mL per every 1 L. As such, to convert between the two measurements, you need to multiply the given volume (0.365 L) by the conversion. To allow for the cancellation of units (liters), liters should be in the denominator of the conversion.
1,000 mL = 1 L
0.365 L 1,000 mL
---------------- x ----------------- = 365 mL
1 L
what is the mole fraction of sulfuric acid in a solution made by adding 3.4 g of sulfuric acid to 3500 ml of water ?
Answers
The mole fraction of sulfuric acid in the solution is 1.78 x 10^-4.
We need to know how many moles of sulfuric acid and how many moles there are in the solution as a whole in order to figure out the mole fraction of sulfuric acid.
To begin, we must convert the sulfuric acid's mass into moles. The molar mass of sulfuric corrosive is 98.08 g/mol. As a result, the number of moles of sulfuric acid is equal to 3.4 g divided by 98.08 g/mol, or 0.0347 mol.
Next, we need to figure out how many moles are all around the solution. We can expect that the volume of the arrangement is equivalent to the volume of water added (3500 ml). Notwithstanding, we want to switch the volume from milliliters over completely to liters since the unit of mole portion is moles per liter.
As a result, the volume of the solution is 3500 ml, or 3.5 L. Based on the assumption that water has a density of 1 g/mL, the mass of water in the solution can be calculated as follows:
The molar mass of water, which is 18.02 g/mol, can be used to determine the number of moles of water: mass of water = volume of water x density of water = 3500 ml x 1 g/mL = 3500 g
The mole fraction of sulfuric acid in the solution can be calculated as follows: 3500 g x 18.02 g/mol = 194.14 mol
Mole fraction of sulfuric acid is calculated by dividing the total number of moles in the solution by the number of moles of sulfuric acid: 0.0347 mol / (0.0347 mol + 194.14 mol) = 1.78 x 10-4.
Therefore, the mole fraction of sulfuric acid in the solution is 1.78 x 10^-4.
For more such questions on mole fraction
https://brainly.com/question/28019441
#SPJ11
A Basketball weighs approximately 2.35 kg. What is the mass of the basketball in grams?
Answers
Answer:
Explanation:
A Basketball Weigh in Grams? A 29.5 inch (75 cm) basketball typically weighs about 624 grams (g). A 28.5 inch (72.4 cm) basketball weighs about 567 grams (g).
wer the following items on a separate piece of paper.
LTIPLE CHOICE
-.Which of the following relationships is true?
A. Higher-energy light has a higher frequency
than lower-energy light does.
B. Higher-energy light has a longer wavelength
than lower-energy light does.
C. Higher-energy light travels at a faster speed
than lower-energy light does.
D. Higher-frequency light travels at a slower
speed than lower-energy light does.
Answers
The answer option which indicates a true relationship is: A. Higher-energy light has a higher frequency than lower-energy light does.
A wave can be defined as a disturbance in a medium that progressively transports energy from its source to another location, without an equivalent transportation of matter.
In Science (Physics), there are two main types of wave:
Mechanical wave.Electromagnetic wave.Light energy is an example of electromagnetic wave because it does not require a medium of propagation for it to travel.
Hence, light energy can travel through a vacuum (space), where no particles exist.
In Science, light energy are grouped into two main categories and these are:
I. Higher-energy light: it has a higher frequency in comparison with others.
II. Lower-energy light: it is typically characterized by a lower frequency.
In conclusion, a higher-energy light has a higher frequency while lower-energy light has a lower frequency.
Read more: https://brainly.com/question/23460034
Answer:
A. Higher-energy light has a higher frequency than lower-energy light does.
Explanation:
Correct Answer
. How many milliliters of 0.20 M HCl are needed to exactly neutralize 40. milliliters of 0.40 M KOH
Answers
Answer:
\(V_{HCl}=80mL\)
Explanation:
Hello,
In this case, for the given reactants we identify the following chemical reaction:
\(KOH+HCl\rightarrow KCl+H_2O\)
Thus, we evidence a 1:1 molar ratio between KOH and HCl, therefore, for the complete neutralization we have equal number of moles, that in terms of molarities and volumes become:
\(n_{HCl}=n_{KOH}\\\\M_{HCl}V_{HCl}=M_{KOH}V_{KOH}\)
Hence, we compute the volume of HCl as shown below:
\(V_{HCl}=\frac{M_{KOH}V_{KOH}}{M_{HCl}} =\frac{0.40M*40mL}{0.20M} \\\\V_{HCl}=80mL\)
Best regards.
Bayer Villiger Provide a balanced chemical equation of the reaction performed in this experiment. Use structures and compound names to show ALL reactants and products involved. Baeyer-Villiger Reaction of Acetophenone Data Results
• Moles of acetophenone used: (Show calculations) 0.020 moles (2.40g/120.151 g mol-1 =0.0199 moles)
• Moles of mCPBA used: (Show calculations) 0.036 moles_(6.25 grams/ 172.56 g.mol-1)
• Expected mass of the product: (Show calculation. Clearly show the limiting and excess reactants)
Answers
Answer:
See the explanations
Explanation:
In the Baeyer-Villiger reaction, we will produce an ester from a ketone (see the first reaction). In our case, the ketone is Acetophenone therefore phenyl acetate would be produced.
Now, for the mass calculation, we have to keep in mind that we have a reaction with a 1:1 ratio. So, if we have 0.02 moles of acetophenone and 0.036 moles of m-CPBA the limiting reagent would be the smallest value in this case acetophenone.
Additionally, if we have a 1:1 ratio and the limiting reagent is 0.02 moles of acetophenone we will have as product 0.02 of phenyl acetate, if we take into account the molar mass of phenyl acetate (136.05 g/mol), we can do the final calculation:
\(0.02~mol~acetophenone\frac{1~mol~phenyl acetate}{1~mol~acetophenone}\frac{136.05~g~phenyl acetate}{1~mol~phenyl acetate}=2.72~g~phenyl acetate\)
I hope it helps!
