An atom of aluminum (AI) has an atomic number of 13 and a mass number of
27. How many neutrons does it have?
Answers
Answer:
14
Explanation:
<3
Related Questions
In an experiment, 24g of magnesium react with 73g of hydrogen chloride to produce a gas and 95g of magnesium chloride.
Write a balanced chemical equation for this reaction
What type of chemical equation for this reaction?
Do these results support the Law of Conservation of Mass Explain your answer.
Answers
Answer:
Mg + 2HCl=== Mg(Cl)2 + H2
The Reaction is a single displacement reaction.
Yes!
This reaction obeys the law of conservation which states that for a closed system... Matter and energy transfer is constant.
Meaning that the Mass of products must equal mass of Reactants
To prove this
lets Calculate the Mass of reactants
24g + 73g=97g.
Mass of Products...
95g + x.
let's calculate the mass of Hydrogen produced.
Note: You can get it using any of the reactants... I'll use Magnesium
Now from the balanced reaction.... 1mole of Mg reacts to produce 1mole of Hydrogen
Moles of Mg=Mass/Molar Mass
Given Mass=24g
Molar mass of Mg=24
Mole of Mg =24/24
=1mole
Since Mole ratio of Mg and H2 is 1:1.... Therefore 1Mole of Hydrogen is produced too.
To get Mass of H2
Again
Mole=Mass/Mm
Known= 1mole of H2 isproduced
Mass of H2 =2g
Mole=Mass/Mm
Mass =Mole x Mm
= 1 x 2
=2g
Now
Total Mass of products is
95 +2 = 97g.
This is the same with the Mass of Reactants
So The Law of Conservation of Mass Holds.
why is it more efficient in a liquid liquid extraction to do multiple extractions rather than one large one
Answers
In liquid-liquid extraction, it is more efficient to do multiple extractions rather than one large one because the solubility of the solute in the solvent may decrease in each extraction.
The amount of solute that dissolves in a solvent decreases with each extraction. Multiple extractions are performed to extract the maximum amount of solute from the mixture being separated in liquid-liquid extraction.
What is liquid-liquid extraction?Liquid-liquid extraction is a technique that is used to isolate one or more dissolved or suspended components from a mixture based on their relative solubilities in two immiscible liquids.
What is multiple extractions?Multiple extractions, also known as re-extraction, is a procedure that involves separating a target compound from a mixture by extracting it several times with the same solvent or a series of solvents.
Multiple extractions are done when the solubility of the solute in the solvent decreases with each extraction. This will help to extract the maximum amount of solute from the mixture.
To know more about multiple extractions click on below link :
https://brainly.com/question/31322526#
#SPJ11
Pls help on test (no old answers OR files)
During a combustion reaction, 9.00 grams of oxygen reacted with 3.00 grams of CH4.
What is the amount of the leftover reactant?
0.74 grams of methane
0.89 grams of methane
1.22 grams of oxygen
1.45 grams of oxygen
Answers
Use the appropriate standard reduction potentials below to determine the equilibrium constant at 391 K for the following reaction under acidic conditions. 41" (aq) + Mn0,(s) +2Fe2+ (aq) --Mn?* (aq) + 2Fe2+ (aq) + 2H,001) Standard reduction potentials: Mnog(x) +44 (aq) +20 - Mn?" (aq) +H,0(1) E - 1.23 V Fe?+ (aq) + 6 Fe?" (ag) = 0.770 V
Answers
Given:
Temperature T = 391KReaction:41" (aq) + Mn0,(s) +2Fe2+ (aq) --Mn?* (aq) + 2Fe2+ (aq) + 2H,001) From the given standard reduction potentials, the balanced chemical reaction taking place can be written as:4H+(aq) + MnO2(s) + 2Fe2+(aq) -> Mn2+(aq) + 2Fe3+(aq) + 2H2O
(l) Standard reduction potentials:
MnO2(s) + 4H+(aq) + 2e- -> Mn2+(aq) + 2H2O(l) E° = 1.23 VFe3+(aq) + e- -> Fe2+(aq) E° = 0.77 V
The balanced chemical reaction can be split into two half-reactions: Oxidation half-reaction: MnO2(s) + 4H+(aq) + 2e- -> Mn2+(aq) + 2H2O(l)Reduction half-reaction: Fe3+(aq) + e- -> Fe2+(aq)The equilibrium constant can be calculated using the Nernst equation, which gives the relationship between equilibrium constants and reduction potentials:log(K) = (nFE°)/2.303RTwhereK = equilibrium constantn = number of electrons transferred in the overall balanced reactionF = Faraday's constantR = gas constantT = temperatureE° = standard reduction potential
We can write the equation for the overall balanced reaction as follows:
Fe3+(aq) + MnO2(s) + 4H+(aq) + 2Fe2+(aq) -> Mn2+(aq) + 2Fe3+(aq) + 2H2O
(l)The standard reduction potential for the overall balanced reaction can be calculated using the standard reduction potentials of the two half-reactions .E° cell = E°(reduction) - E°(oxidation)E° cell = 0.77 V - 1.23 V = -0.46 V Substituting the values in the Nernst equation: log(K) = ((2)(96485 C mol-1)(-0.46 V))/(2.303(8.314 J mol-1 K-1)(391 K))log(K) = -9.7K = 2.0 x 10-10Therefore, the equilibrium constant at 391 K is 2.0 x 10-10.
To know more about half-reactions refer to:
https://brainly.com/question/26411933
#SPJ11
Calculate the relative formula mass of strontium nitrate, Sr(NO3)2.
(relative atomic masses: N = 14, O = 16, Sr = 88)
Answers
Answer:
its 210
Explanation:
Just add all atomic mass used in the formula together: 2*(14+16*3)+88= 2*62+88=124+88=210
Hope this was helpful
The relative formula mass of strontium nitrate Sr(NO₃)₂ is 210
The relative atomic masses of N, O and Sr are 14,16 and 88 respectively.
In calculating the relative atomic mass of an element with isotopes, the relative mass and proportion of each is taken into account. Adding the atomic masses together gives the relative formula mass of a compound
So, relative atomic mass of Sr(NO₃)₂ is calculated as
88+ 2(14+16×3) = 210
The atomic mass constant (symbol: mu) is defined as being 1/12 th of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless; hence the value is said to be relative atomic mass.
To know more about strontium nitrate here
https://brainly.com/question/26177156
#SPJ2
Look at the picture
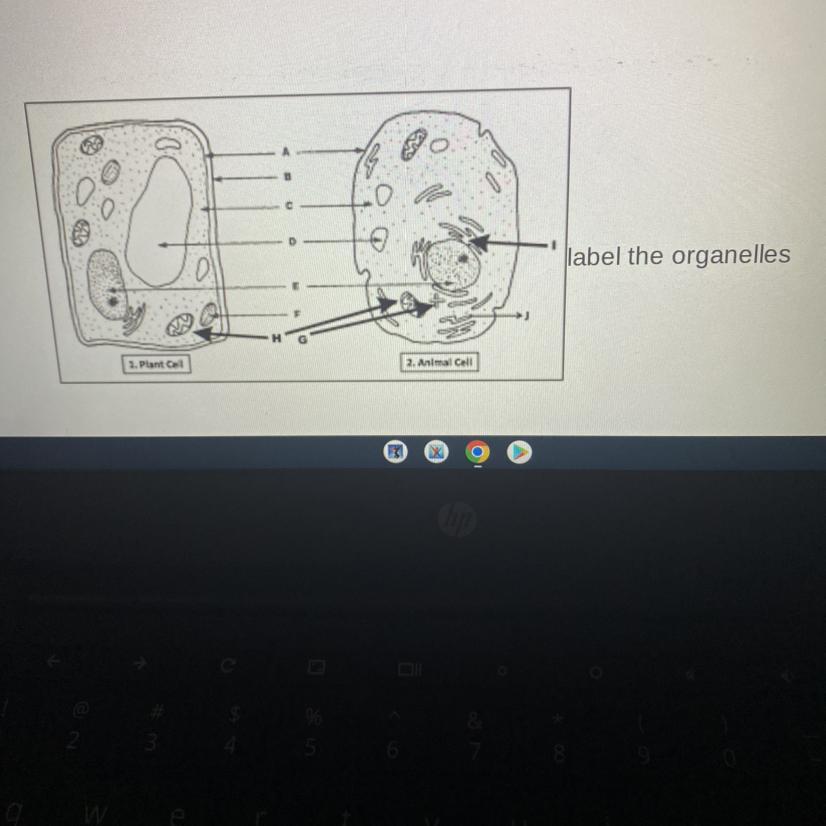
Answers
Answer:
A: cell membrane
B: cell wall
C: cytoplasm
D: vacuole
E: Nucleus
F: if it's green, it's chloroplast
G: probably centrioles
H: I think mitochondria
Explanation:
checked with my notes and with the internt to be sure that it's mostly correct.
This is hard to learn, you need to memorize it. It's way easier to know how the part looks like and what it does than knowing the name and not knowing what it's function.
Good luck
which of the following is not true about a spontaneous process? a) a spontaneous process is one that continues on its own once begun. b) spontaneous processes occur naturally. c) a nonspontaneous process does not occur unless some external action is applied. d) a nonspontaneous process does not mean it cannot ever occur under any conditions. e) a spontaneous process is also spontaneous in the reverse direction.
Answers
The statement a spontaneous process is also spontaneous in the reverse direction is not true about a spontaneous process (Option e).
What is a spontaneous process?A spontaneous process in physics makes reference to any phenomenon in which it can be triggered naturally without requiring an input of energy to carry out, which occurs when continues on its own once begun.
Therefore, with this data, we can see that a spontaneous process in physics refers to all phenomena that may progress with the need of a given input of energy to be produced.
Learn more about a spontaneous process here:
https://brainly.com/question/2855292
#SPJ1
Why does electron affinity tend to become more exothermic as you move right across a period? a. The trend in electron affinity is just the opposite of that for first ionization energy, since the two processes are the oppose of each other. b. As you move right across the period, you have a greater likelihood of pairing electrons in an orbital, which is a more stable configuration. c. As you move right across the period, metallic character decreases, meaning that the atom or ion is more likely to gain electrons than lose electrons. d. The effective nuclear charge increases as you move right across the period, resulting in greater attraction for an electron.
Answers
Electron affinity tend to become more exothermic as you move right across a period because the effective nuclear charge increases as you move right across the period, resulting in greater attraction for an electron. The correct answer is: D.
Electron affinity is the energy change that occurs when an electron is added to a neutral atom in the gaseous state. It is measured in kJ/mol and is typically reported as a positive or negative value.
A positive electron affinity means that energy is required to add an electron to the atom, while a negative electron affinity means that energy is released when an electron is added to the atom.
The effective nuclear charge is the net positive charge experienced by an electron in an atom. It is calculated by subtracting the number of shielding electrons from the number of protons in the nucleus.
As you move right across a period, the effective nuclear charge increases because each successive element has one more proton in its nucleus.
This increase in effective nuclear charge results in a greater attraction for an electron, which makes it more likely for an atom to gain an electron.
Therefore, electron affinity tends to become more exothermic as you move right across a period because the effective nuclear charge increases, resulting in greater attraction for an electron.
To know more about Electron affinity, refer here:
https://brainly.com/question/977718#
#SPJ11
What does the author predict about the future of the Earth's mantle? Do you support his educated guess? Use complete sentences to answer.
Answers
The earth's mantle will not change in the future because this region is safe from the intervention of human beings.
What happens in the Earth's mantle?The transfer of heat and material in the mantle helps us to determine the landscape of Earth. Activity in the mantle moves the plate tectonics, that contributes to volcanoes, seafloor spreading, earthquakes, and building of mountains.
So we can conclude that the earth's mantle will not change in the future because this region is safe from the intervention of human beings.
Learn more about mantle here: brainly.com/question/349072
#SPJ1
1. Amy's science teacher shows her class a model of an atom. Why is a model useful for studying atoms?
A. All atoms exist in the gas phase, and atmospheric gases are invisible.
B. A model is helpful to observe the behavior of the parts of an atom.
C. The parts of an atom cannot be directly observed.
D. A model of an atom is the same for all atoms.
Answers
Answer:
The parts of an atom cannot be directly observed.
Explanation:
An atom is the smallest indivisible particle of a substance that can take part in a chemical reaction.
Atoms are not observed with naked eyes because they are found in the realm of very small particles. It is impossible to observe the parts of an atom directly.
As a result of this, models suffice in explaining the parts of an atom. Hence the answer.
What are the current and or future uses of genetically modified strawberries
Answers
In the future, genetically modified strawberries may become more widely available if they pass regulatory approvals and are deemed safe for consumption. They could potentially provide benefits such as reduced pesticide use, longer shelf life, and improved nutrition. However, there are also concerns about the environmental impact and potential health risks associated with genetically modified crops, which will need to be addressed before they can be widely adopted.
gallium-67 is used medically in tumor-seeking agents. the half-life of gallium-67 is 78.2 hours. if you begin with 51.3 mg of this isotope, what mass remains after 127 hours have passed? mg
Answers
The decay of Gallium-67 follows the exponential decay formula:
\(N = N_0 * (1/2)^(^t^/^T^1^/^2^)\)
Where:
N = final amount remaining
\(N_0\)= initial amount
t = time elapsed
\(T_1_/_2\) = half-life
The first thing we can do is find out how many half-lives have passed in 127 hours:
Number of half-lives = t / T1/2 = 127 hours / 78.2 hours = 1.624
Next, we can determine how much Gallium-67 is left using this value:
Rounding to the nearest decimal point, N = N0 * (1/2) * (t/T1/2) = 51.3 mg * (1/2) * (1.624) = 25.6 mg.
Therefore, after 127 hours about 25.6 mg of gallium-67 remains.
Learn more about half -lives, here:
https://brainly.com/question/30599798
#SPJ1
the pKa of N-benzyl-1,1-diphenylmethanimine (imine) is?
Answers
The pKa of N-benzyl-1,1-diphenylmethanimine (imine) is not readily available in the literature,it is important to note that imines, in general, have a pKa range of 5-7 depending on the substituents attached to the nitrogen atom.
However, it is important to note that imines, in general, have a pKa range of 5-7 depending on the substituents attached to the nitrogen atom. The presence of the benzyl and diphenyl groups in N-benzyl-1,1-diphenylmethanimine may affect its pKa compared to other imines.
It is also important to note that the pKa value of a compound indicates the acidity or basicity of the molecule, which affects its reactivity and solubility properties.
Therefore, knowing the pKa value of N-benzyl-1,1-diphenylmethanimine can provide important information for synthetic chemists when designing and optimizing reaction conditions.
The pKa of N-benzyl-1,1-diphenylmethanimine, which is an imine, refers to the acidity constant of the compound. Imines are compounds featuring a carbon-nitrogen double bond, and in this specific case, N-benzyl-1,1-diphenylmethanimine is characterized by a benzyl group and two phenyl groups connected to the imine carbon. However, It is bit difficult to provide a specific pKa value for N-benzyl-1,1-diphenylmethanimine as it appears that the experimental value for this particular compound is not readily available in the literature.
Typically, imines have pKa values in the range of 9-11. The specific pKa value depends on the electronic and steric effects of the substituent groups on the imine nitrogen and carbon. In the case of N-benzyl-1,1-diphenylmethanimine, the presence of the benzyl and diphenyl groups could potentially influence the pKa value, making it different from other imines. To determine the exact pKa value for this compound, one would need to either consult specialized literature or perform experimental measurements.
To know more about imines: brainly.com/question/22944136
#SPJ11
Explaining How Rocks are formed
Rock Cycle in Earth's Crust
Question 1
6 pts
For the Rocks pictured in #4 today, which types of rocks (A, B, or C) did you say each rock (#1, #2,
and #3) were? Explain why you chose what you chose. (1 point each for the label of Type A, B, or C
to Rock #1, 2, or 3; and 1 point each for explanation of why these were chosen.)
Edit View Insert Format Tools Table
12pt v
Paragraph
B I I o Ауру т?v
:
Answers
There are three kinds of rock cycle in Earth's Crust:
Igneous rocks form when molten rock (magma or lava) cools, crystallizes after that solidifies. Sedimentary rocks originate from pre-existing rocks or pieces of once-living organisms. It's happen when particles settle out of water or air, or by precipitation of minerals from water. Metamorphic rocks form when rocks are subjected to high heat, high pressure, hot mineral-rich fluids or some combination of these factors.Rocks is one of the main raw materials of the Earth's crust. This is because the rock that decomposes becomes soil which is used as a medium for plant growth and as a place to live for all living things on Earth. There are three kinds of rocks that exist on Earth with a long process of formation. Each of these rocks forms a layer in the Earth's atmosphere with different shapes and textures.
Learn more about the rock layers:
https://brainly.com/question/25846908
#SPJ11
people tint their car windows to stop what

Answers
Answer:
Reflection
Explanation:
Answer:
b
Explanation:
describe the Solvay process
Answers
CaC2 + 2H2O → C2H2 + Ca(OH)2If 4.8 moles of CaC2 are consumed in this reaction, how many grams of H2O are needed?
Answers
The given reaction is already balanced, that is to say tha the number of atoms in the reactants matches the number of atoms in the products. In the reaction, we can see the relationship between CaC2 and H2O. For each mole of CaC2 two moles of H2O react.
So, if 4.8 moles of CaC2 are consumed the moles of H2O needed will be:
Mol of H2O = Mol of CaC2 x 2
Mol of H2O = 4.8 x 2 = 9.6 mol of H2O
Now, to calculate the grams of H2O we will use the following equation and the mass molar of H2O.
Mass molar of H2O =18.01 g/mol
\(\begin{gathered} \text{Mass of H2O=Mol of H2O }\times Mass\text{ molar of H2O} \\ \text{Mass of H2O = 9.6 mol }\times18.01\frac{\text{ g}}{mol} \\ \text{Mass of H2O = 172.9 g} \end{gathered}\)So, if 4.8 moles of CaC2 are consumed in this reaction, 172.9 g of H2O are needed
The formula of the gas ozone is O 3. What is the volume of 48g of ozone at r.t.p?
Answers
Answer:
1.8 x 10^ 24 atoms of oxygen
Explanation:
The molecular weight of ozone is known to be 48 grams / mol. Here we are given a sample of 48 grams of ozone as well, so in 48 grams of ozone the number of moles = 48 / 48 = 1,
_______________________________________________________
1 mole of ozone is equal to 6.0221415 × 10^23 molecules of ozone. Respectively, 1 molecule of ozone has 3 atoms of oxygen. Thus, you can conclude the following -
3 * 6.0221415 × 10^23 = ( About ) 1.8 x 10^ 24 atoms of oxygen
Hope that helps!
The volume of 48 grams of ozone at room temperature and pressure (r.t.p) is equal to 24 \(dm^3\).
Given the following data:
Mass of ozone = 48 grams.Scientific data:
Molar mass of ozone = 48 g/mol. Avogadro's number = \(6.02 \times 10^{23}\)To determine the volume of 48 grams of ozone at room temperature and pressure (r.t.p):
First of all, we would calculate the number of moles of ozone contained in 48 grams of ozone by using the formula:
\(Number\;of\;moles = \frac{mass}{molar\;mass}\\\\Number\;of\;moles = \frac{48}{48}\)
Number of moles = 1.0 moles
By stoichiometry:
1 mole of ozone = 24 \(dm^3\)
Note: At room temperature and pressure (r.t.p), the volume of any gas is equal to 24 \(dm^3\) or 24,000 \(cm^3\).
In conclusion, the volume of 48 grams of ozone at room temperature and pressure (r.t.p) is equal to 24 \(dm^3\).
Read more on moles here: Read more: https://brainly.com/question/16906167
50 points Use the drop-down menus to select the names of the labeled structures.
A:
B:
C:
D:

Answers
Answer:
A: PistilB: sepalC: petal
D:stamen

Answer:
Pistil
sepal
petal
stamen
How many moles of potassium chloride are needed to react with 9. 27 moles of
oxygen gas?
2KCI (s) + 302 (g) - — 2KCIO3 (s)
Answers
To determine the number of moles of potassium chloride (KCl) required to react with 9.27 moles of oxygen gas ( O_{2}), we need to use the stoichiometry of the balanced chemical equation. The balanced equation shows that 2 moles of potassium chloride react with 3 moles of oxygen gas to produce 2 moles of potassium chlorate (\(KClO_{3}\)).
According to the stoichiometry of the balanced chemical equation, 2 moles of potassium chloride react with 3 moles of oxygen gas to produce 2 moles of potassium chlorate. Therefore, we can set up a ratio based on this stoichiometry:
2 moles KCl / 3 moles O_{2}= x moles KCl / 9.27 moles O_{2}
Solving for x, we can find the number of moles of potassium chloride required:
x = (2 moles KCl / 3 moles O_{2}) * 9.27 moles \(O_{2}\)
x = 6.18 moles KCl
Therefore, 6.18 moles of potassium chloride are needed to react with 9.27 moles of oxygen gas. The stoichiometry of the balanced equation allows us to determine the appropriate amounts of reactants required for the given reaction.
Learn more about stoichiometry here: https://brainly.com/question/14935523
#SPJ11
pleaseee help ASAP
will mark brainliest!!! <3

Answers
explanation:

Answer:
it is C
Explanation:
I saw from Google. Hope it helps.
I cant get the full picture
sorry

A higher-frequency wave has more energy than a lower-
frequency wave with the same amplitude.
Answers
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
write the empirical formula for at least four ionic compounds that could be formed from the following ions: o CIO3^-,
o nh4^ ,
o ch3co2^-,
o pb^4+
Answers
Empirical formulae are the ratios of atoms in a compound that show the lowest possible ratio of atoms per mole of compound.
What is the empirical formula?In this question, we are to find the empirical formulae for the following ions:
OCl³⁻, NH⁴⁺, CH₃CO₂⁻ and Pb⁴⁺
Empirical formula for OCl³⁻:Oxygen (O) forms an anion by gaining three electrons to form the oxide ion, O²⁻
The ClO₃⁻ ion has one O²⁻ and three Cl⁺ ions. Therefore, the empirical formula is OCl³⁻
Empirical formula for NH₄⁺ :Nitrogen (N) forms a cation by losing three electrons to form N₃⁺ while Hydrogen (H) forms a cation by losing an electron to form H⁺. Therefore, the empirical formula for NH₄⁺ is NH₄⁺
Empirical formula for CH₃CO₂⁻
The ion contains 2 Carbon atoms, 3 Oxygen atoms, and 4 Hydrogen atoms. Divide each number of atoms by the lowest number (2) to give the empirical formula: CH₃CO₂⁻
Empirical formula for Pb⁴⁺:Lead (Pb) forms a cation by losing four electrons to form Pb⁴⁺.Therefore, the empirical formula for Pb⁴⁺+ is Pb⁴⁺.
Learn more about Empirical formula here:
https://brainly.com/question/14044066
#SPJ11
Express the composition of each compound as the
mass percent of its elements (percent composition).
a.
sucrose (C,H),0.,)
c. magnetite (Fe,O.)
b.
aluminum sulfate
(Al, (SO,).)
Answers
The composition of each compound as the mass percent of its elements is as follows:
For Sucrose - Carbon is 42.18%, Hydrogen is 6.44% and Oxygen is 51.38%.For Aluminum sulfate - Aluminum is 3.99%, Sulfur is 4.57% and Oxygen is 87.44%.For Magnetite - Iron is 71.83% and Oxygen is 28.17%.What is mass percent?Concentration is expressed as mass percent. Additionally, the ingredients in a particular mixture are described. Solution composition can be understood in terms of mass percent. It indicates the mass of solute present in a solution of a given mass. The amount of solute is expressed in mass or moles.
Mass Percent = (Component Mass ÷ Total Mass) x 100% or
(mass of solute ÷ mass of solution) x 100%
a. Sucrose (C₁₂H₂₂O₁₁) with molecular mass of 342.296 u composed of:
Carbon: (12 x 12.01) / 342.296 = 42.18%
Hydrogen: (22 x 1.01) / 342.296 = 6.44%
Oxygen: (11 x 16) / 342.296 = 51.38%
b. Aluminum sulfate (Al₂(SO₄)₃) with molecular mass of 342.14 u is composed of:
Aluminum: (2 x 26.98) / 342.14 = 3.99%
Sulfur: (3 x 32.06) / 342.14 = 4.57%
Oxygen: (18 x 16) / 342.14 = 87.44%
c. Magnetite (Fe₃O₄) with molecular mass of 231.534 u is composed of:
Iron: (3 x 55.85) / 231.534 = 71.83%
Oxygen: (4 x 16) / 231.534 = 28.17%
To know more about mass percent, visit:
https://brainly.com/question/5840377
#SPJ1
The value of resistance r was determined by measuring current I flowing through the resistance with an error
Answers
Correct question is;
Resistance of a given wire is obtained by measuring the current flowing in it and the voltage difference applied across it. If the percentage errors in the measurement of the current and the voltage difference are 3% each, then error in the value of resistance of the wire is?
Answer:
6%
Explanation:
From ohms law, we know that;
R = V/I
Where;
R is resistance
V is voltage
I is current
Now, the percentage error in the resistance is given by the formula;
ΔR/R = ΔV/V+ ΔI/I
We are told that the current and the voltage difference have a percentage error of 3% each. Thus;
ΔR/R = 3% + 3% = 6%
Calculate the hydrogen ion concentration of a Nitric acid solution with a pH of 2.11.
[H] = _____ (round to 2 decimal places)
Answers
Answer:
0.01 MExplanation:
The hydrogen ion concentration can be found by finding the antilog of the pH
\(pH = - log [ {H}^{+} ]\)
We have
\(2.11 = - log({H}^{+}) \\ find \: \: antilog \: of \: both \: \: sides \\ \\ |{H}^{+}| = {10 }^{ - 2.11} \\ = 0.00776...\)
We have the final answer as
0.01 M to 2 decimal places
Hope this helps you
things that happened to the organisms I tracked: (example: was eaten by other organisms)
Answers
Reproduced, transferred their genes to progeny, died from natural causes, fell ill or contracted a disease, became prey for a predator or were devoured by a scavenger, or moved to a new area.
What kind of non-living entity might be present in an ecosystem?Non-living things include items like rocks, water, the atmosphere, the climate, and natural occurrences like earthquakes and rockfalls. One of the qualities that characterises living beings is their capacity for reproduction.
What are five non-living examples?Its definition includes glass, the sun, water, sand, and rock as non-living objects. They show absolutely no signs of life. Some people define a non-living object as anything that once belonged to a live entity.
To know more about scavenger visit:-
https://brainly.com/question/31224075
#SPJ1
Why is it necessary to adjust the height of the graduated cylinder when measuring the volume of co2 (g) produced?.
Answers
It is necessary to adjust the height of the graduated cylinder when measuring the volume of co2 (g) produced to maintain atmospheric pressure.
Pressure is defined as force/area. To illustrate the pressure from snow on a roof, divide the weight of the snow by the surface area of the roof. Gases are a typical pressure source in chemistry. A "vacuum" is used to describe the absence of pressure. Humans have long held the belief that vacuums are both improbably rare and unnatural since "nature abhors a vacuum." Actually, this is not the case.
The number of pressure units is ridiculous. It's common to use the torr or mmHg unit. This discussion is solely focused on the height of a mercury column. The atmosphere contains 760 torr, or mmHg. You could also look at mmH2O, which makes use of a comparable idea.
To know more about pressure visit : brainly.com/question/18124975
#SPJ4
If 77.0 mL of nitrogen gas is collected over water at 50 °C and 763 mm Hg, what is the volume of dry nitrogen gas at STP? The vapor pressure of water at 50 °C is 92.5mm Hg.
Answers
The volume of the dry nitrogen gas at STP is 57.4 mL
From the question given above, the following data were obtained:
Initial volume (V₁) = 77 mL
Initial temperature (T₁) = 50 °C = 50 + 273 = 323 K
Initial pressure (P₁) = 763 – 92.5 = 670.5 mmHg
Final temperature (T₂) = STP = 273 K
Final pressure (P₂) = 760 mmHg
Final volume (V₂) =?The volume of the dry nitrogen can be obtained by using the combine gas equation as follow:
\( \frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2} \\ \\ \frac{670.5 \times 77}{323} = \frac{760 \times V_2}{273} \\ \\ cross \: multiply \\ \\ 323 \times 760 \times V_2 = 670.5 \times 77 \times 273 \\ \\ divide \: both \: side \: by \: 323 \times 760 \\ \\ V_2 = \: \frac{670.5 \times 77 \times 273 }{323 \times 760} \\ \\ V_2 = 57.4 \: ml\)
Therefore, the volume of the dry nitrogen gas is 57.4 mL
Learn more: https://brainly.com/question/25080013