Answers
Answer:
Chemical
Explanation:
It's rust being chemically bleached away
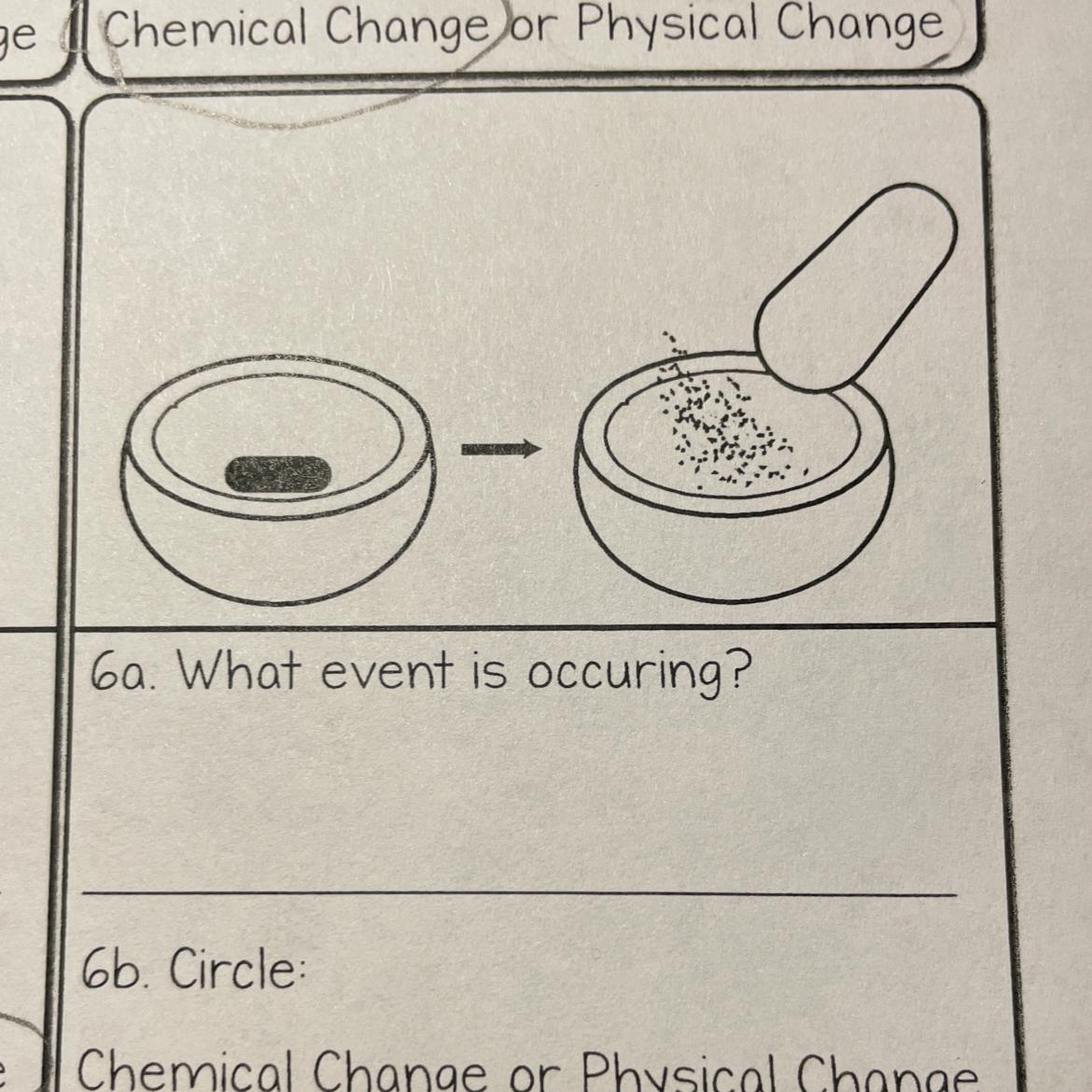
Answer:
physical, it's just being made into smaller pieces. doesn't change color or chemically change
Answer is Physical Change
Related Questions
1. (04.01 LC)
Which of the following is an example of how science can solve social problems? (5 po
It cathstop excessive rain from occurring.
It can identify the sources of polluted water.
It can control the time and day when cyclones happen.
It can reduce the frequency of severe weather conditions.
2. (04.01 LC)
Answers
Science is used to stop things the are incrediblu diffucult to deal with therefore jeg spiser ikke dreng
The answer is
It can identify the sources of polluted water.
What’s the missing word

Answers
Answer:
crystallized by heating the salt solution
Salt solutions can be crystallized to give solid salts.
the smallest representative unit of an ionic compound is called a ?
Answers
The lowest representational unit for an ionic compound is a formula unit.
What does a chemical representative unit mean?The smallest unit in which a substance naturally exists is called a representative particle. The atom is the representative particle for most elements. The atom is the representative particle for most elements. Iron, carbon, and helium are made up of individual iron, carbon, and helium atoms.
What are atoms smaller than?Subatomic is defined as "less than an atom." Protons, neutrons, and electrons make up atoms. Even smaller particles known as quarks are the building blocks of protons and neutrons. Physicists believe quarks are elementary particles based on the evidence that is currently available.
To learn more about ionic compound visit:
brainly.com/question/29005103
#SPJ4
what is the de Broglie wavelength of an electron traveling at 1.48 x 10^5 m/s?

Answers
The formula of De Broglie is the following:
\(\lambda=\frac{h}{mv}\begin{cases}\lambda=wavelength\text{ (m)} \\ h=Planck\text{ constant =6.626}\cdot10^{-34}Js \\ m=mas\text{s (kg)} \\ \text{v=}velocity\text{ (m/s)}\end{cases},\)Remember that the mass of an electron is 9.11 x 10 ^(-31) kg, so replacing in the formula, we're going to obtain:
\(\begin{gathered} \lambda=\frac{6.626\cdot10^{-34}J\cdot s}{9.11\cdot10^{-31}\operatorname{kg}\cdot1.48\cdot10^5\frac{m}{s}}, \\ \lambda=4.91\cdot10^{-9}\text{ m} \end{gathered}\)The answer is that the wavelength of an electron traveling at 1.48 x 10^(5) m/s is 4.91 x 10^(-9) m.
Why can you find different features at an oceanic convert zone than those found in continental convert zone
Answers
Answer:
Because both the zones have different compositions.
Explanation:
The continental crust is composed of mainly graphite whereas the oceanic crust is made up of basalt. The oceanic plates are thinner than the continental plates. Also the oceanic plates are much denser as compared to the continental plates. Now the continental plates at the convergent boundary gained thickness as they are pushed upward. These plates or zones are of different composition an densities and so when they collide different features can be seen at the two conversion zones.
Calculate the amount of energy required to boil 25.00 g of mercury.

Answers
Answer:
C. 7.4 kJ
Explanation:
Let assume that Mercury is at room temperature (25 °C). The energy required to boil the sample of mercury is the sum of sensible and latent heats. Mercury has a fussion and boiling points of -38.83 °C and 356.7 °C, respectively, and a specific heat of \(138\,\frac{J}{kg\cdot ^{\circ}C}\). Then:
\(Q = (25\,g)\cdot \left[\left(0.138\,\frac{J}{g\cdot ^{\circ}C} \right)\cdot (356.7^{\circ}C - 25^{\circ}C) + 296\,\frac{J}{g} \right]\)
\(Q = 8544.365\,J\)
The option that offers the best approximation to the result is C.
T or F: Lone pairs around the oxygen atom of a water molecule play no role in determining its molecular geometry?
Answers
Answer:
Explanation:
They play a very important part. The geometry is not a straight line. It is an angle over 90 which means that the molecule has the same general shape as a boomerang. The two hydrogens and the 2 lone electron pairs try to get away as far as possible from each other. The actual shape results in a tetrahedron shape. But the two hydrogens and 1 oxygen actually look like the aforementioned boomerang.
an unknown quantity of gas held at 1195 K in a container with a volume of 27 L and a pressure of 1.65 atm. How many moles of gas are in the sample?
Answers
The number of moles of the gas present in the container at 1195 K and pressure of 1.65 atm is 0.45 mole
How do i determine the number of mole present in the container?From the question given above, the following data were obtained obtained:
Temperature (T) = 1195 KVolume of container (V) = 27 L Pressure (P) = 1.65 atmGas constant (R) = 0.0821 atm.L/mol KNumber of mole (n) =?Using the ideal gas equation, we can obtain the number of mole present in the container as follow:
PV = nRT
1.65 × 27 = n × 0.0821 × 1195
44.55 = n × 98.1095
Divide both sides by 98.1095
n = 44.55 / 98.1095
n = 0.45 mole
Thus, we can conclude that the number of mole of the gas present in the container is 0.45 mole
Learn more about number of mole:
https://brainly.com/question/29927685
#SPJ1
Why did the toads disappear
Answers
Answer: extremely sensitive to environmental changes caused by habitat destruction, climate change, pollution
Explanation:
Across the world, amphibians have been disappearing at an unprecedented rate — faster than any other group of animals. Frogs, toads, and salamanders are extremely sensitive to environmental changes caused by habitat destruction, climate change, pollution, and invasive species.
Which is the best definition of
force?
A. a push or pull
B. a change in motion
C. a motion that does not change
Answers
Answer:
A
Explanation:
........................
Answer:
push or pull i think..okay?
Which location below would be considered a microhabitat
Answers
Answer: An overturned decomposing log or the underside of a rock in a stream are excellent examples of microhabitats. Both a habitat and a microhabitat have typical abiotic (e.g. water, temperature, light.
Explanation:
If the experiment is repeated at ph 11, the observed activity level of the enzyme will most likely be:
Answers
If the experiment is repeated at the pH 11 level, the enzyme activity will be less than at the pH 9 level.
We can arrive at this answer because:
Through the graph, we can see the level of enzyme activity in relation to pH.From pH 5, we can see that as the pH goes up, the enzyme activity goes down.In this case, we can say that if the experiment is repeated at pH 11, the enzyme activity level will be even lower, since from pH 5 onwards, as the pH rises, the enzyme activity goes down.
The question above is incomplete, however, you can see a full version of it in the image below.
More information:
https://brainly.com/question/19840255?referrer=searchResults

chlorine gas can be made from the reaction of manganese dioxide with hydrochloric acid. what is the theoretical yield of cl2 when 28 g of mno2 are mixed with 42 g of hcl?
Answers
Given, Manganese dioxide (MnO2) = 28gHydrochloric acid (HCl) = 42g
To find, Theoretical yield of Cl2, We will find the limiting reagent and use its mole to find the theoretical yield of Cl2.MnO2 + 4HCl → MnCl2 + 2H2O + Cl2First we will find the limiting reagent.
The number of moles of MnO2 is, Moles of MnO2 = Mass/Molar mass = 28/86.94 = 0.322 mol The number of moles of HCl is, Moles of HCl = Mass/Molar mass = 42/36.46 = 1.151 mole will use the mole ratio of MnO2 and HCl to find the mole of Cl2. The mole of HCl required to react with 0.322 mol of MnO2 = 0.322 x 4 = 1.288 mol The number of moles of Cl2 will be, Moles of Cl2 = 0.322 x 1 / 4 = 0.0805 mol. The theoretical yield of Cl2 from the given reactants is,
Theoretical yield of Cl2 = Moles x Molar mass = 0.0805 x 70.9 = 5.71 g Chlorine gas will be produced with a theoretical yield of 5.71 g when 28 g of MnO2 and 42 g of HCl are mixed.
Learn more about theoretical yield
https://brainly.com/question/25996347-
#SPJ11
explain why chlorine does not react with aqueous sodium fluorine
Answers
Answer:
because the two are opposite compounds.
Explanation:
When Chlorine is passed through aqueous sodium fluorine then no reaction takes place between these two because of reactivity order of Fluorine and Chlorine
What is displacement reaction ?Displacement reaction is a reaction in which one atom replaces other atom from a molecule on the basis of reactivity strength.
The reason for such reactivity is that as we go down the group the number of shells increases so outermost shell is very far away from the nucleus. If any atom from outside coming to this nucleus then this nucleus is unable to attract the electron of that that atom. Reactivity of halogens is directly proportional to the electronegativity power of that element, that is power to attract electron. Reactivity of halogens decreases down the group.
So Chlorine is less reactive than Fluorine so Chlorine can not replace Fluorine from sodium fluoride. Hence no reaction will take place.
To learn more about displacement reaction, here:
https://brainly.com/question/20690229
#SPJ2
A. How well do the continents fit together
Answers
Answer:
The shapes of continents fit together like a puzzle.
Explanation:
Just look at the east coast of South America and the west coast of Africa—it's almost a perfect fit! Identical rocks have been found on different continents. These rocks formed millions of years ago before the continents separated.
How does the size of ice affect the rate of melting?
Answers
The larger ice cubes require more heat from the water to melt. To transfer more heat from the water requires more time. Therefore, it takes longer for the larger ice cubes to melt.
A 100. 0 mL sample of natural water was titrated with NaOH. The titration required 13. 57 mL of 0. 1123 M NaOH solution to reach a light pink phenolphthalein end point. Calculate the number of millimoles of NaOH required for the titration
Answers
A 100.0 mL sample of natural water was titrated with 13.57 mL of 0.1123 M Na OH solution to reach a light pink are the phenolphthalein end point. The number of millimoles of Na OH required for the titration is 1.525011 millimoles. Titration is a technique used in chemistry
to identify the quantity of a substance by adding a reactant until the chemical reaction is completed. In titration, a solution of known concentration (the titrant) reacts with a solution of unknown concentration (the analyte) to determine its concentration. Titration of natural water with Na OH In this case, we are titrating natural water with Na OH to find the concentration of the unknown solution. The balanced chemical reaction for the titration of natural water with Na OH is:H2O + Na OH → Na+ + OH- + H2O
The volume of NaOH required to reach the end-point of the titration is 13.57 mL. The molarity of Na OH used for the titration is 0.1123 M. We can use the following formula to calculate the number of millimoles of Na OH required for the titration Millimoles of Na OH = (Volume of Na OH × Molarity of NaOH) / 1000Substitute the given values in the above equation and solve for the millimoles of Na OH required for the titration. Millimoles of Na OH = (13.57 mL × 0.1123 M) / 1000= 0.001525011 millimoles Therefore, the number of millimoles of NaOH required for the titration is 1.525011 millimoles.
To know more about chemistry Visit;
https://brainly.com/question/14329098
#SPJ11
6. India is a country connected to the continent of Asia. India used to be far apart
from Asia. Which diagram below shows what happened to the plates that India
and Asia are part of?
Answers
Which describes the oxidizing agent in a chemical reaction?
the substance that is oxidized because it loses electrons
the substance that is reduced because it loses electrons
the substance that is oxidized because it gains electrons
the substance that is reduced because it gains electron
Answers
The oxidizing agent are the substance that is reduced because it gains electrons.
What is an oxidizing agent?An oxidizing agent is defined as the agent that is capable of undergoing oxidation reaction by losing an electron.
The following are the characteristics of an oxidizing agent:
At the end of the chemical reaction they are reduced,They gain electrons during chemical reaction.They can easily transfer their oxygen atoms.Learn more about electrons here:
https://brainly.com/question/1390694
#SPJ1
Answer:
D: the substance that is reduced because it gains electrons.
Explanation:
1. Name the elements with the following symbols
a. Na
b. H
c. C
d. O
2. What is the symbol for the following elements?
a. Helium
b. Sulfur
c. Copper
d. Lead
Answers
Answer:
1.
a. Sodium
b.Hydrogen
c Carbon
d Oxygen
2.
a He
b S
c Cu
d Pb
Answer:
1.
a. Sodium
b.Hydrogen
c Carbon
d Oxygen
2.
a He
b S
c Cu
d Pb
Explanation:
Hope this will help
2017 chemistry corner: chemical reactions: types of reactions worksheet
Answers
There are different types of reactions which are classified based on the mode of regrouping of atoms between the reactants. Some of the reaction types are synthesis, decomposition, displacement etc.
What are the types of reactions?There are different types of reaction based on the regrouping of atoms to form a new product. Some of them are described below:
Synthesis reactions:
This type of reactions involves combination of two reactants to produce a single product as follows:
A + B →C
Decomposition reactions:
Decomposition of a compound into its constituent elements or compounds as written below:
AB₂ → A + 2B
Displacement reaction:
In displacement reactions, one or two group of the reactants are displacing each other.
AB + CD → AC + BD - double displacement
AB + C → AC + B - single displacement.
Combustion reaction:
This reaction is the reaction with oxygen forming carbon dioxide and water.
Find more on types of reactions:
https://brainly.com/question/14459742
#SPJ1
Which of the following is the most likely description of the product or
products of a double-displacement reaction?
A.one new compound and an element
B.two new compounds
C.one new compound
D.oxygen and energy
Answers
Two new compounds are the most likely description of the products of a double-displacement reaction (Option B).
What is a double displacement chemical reaction?A double displacement chemical reaction can be defined as a reaction where ions present in two different compounds react in order to generate one or more new compounds which are present in an aqueous media.
Therefore, with this data, we can see that a double displacement chemical reaction is based on the reaction of ionic compounds in order to generate two or more new compounds in water.
Learn more about a double displacement chemical reaction here:
https://brainly.com/question/26413416
#SPJ1
convert 125.0g H2O to molecules
Answers

Plants use sunlight as an energy to convert carbon dioxide and water into glucose and oxygen which best describes the reaction
Answers
Answer:
photosynthesis
Explanation:
Answer:its part of the process of photosynthesis
Explanation:
Provide 4 examples of each of the following, what are they used for and their environmental health and safety impacts: - Natural Nanomaterial - Engineered Nano materials - Organic Nano materials - Inorganic Nanomaterials
Answers
Nanomaterials, whether natural, engineered, organic, or inorganic, offer various applications across industries. However, their environmental health and safety impacts need to be carefully evaluated and managed to mitigate any potential risks.
Understanding their properties, fate, and behavior in different environments is crucial for responsible development, use, and disposal of nanomaterials.
Natural Nanomaterials:
Examples: Carbon nanotubes (CNTs) derived from natural sources like bamboo or cotton, silver nanoparticles in natural colloids, clay minerals (e.g., montmorillonite), iron oxide nanoparticles found in magnetite.
Uses: Natural nanomaterials have various applications in medicine, electronics, water treatment, energy storage, and environmental remediation.
Environmental health and safety impacts: The environmental impacts of natural nanomaterials can vary depending on their specific properties and applications. Concerns may arise regarding their potential toxicity, persistence in the environment, and possible accumulation in organisms. Proper disposal and regulation of their use are essential to minimize any adverse effects.
Engineered Nanomaterials:
Examples: Gold nanoparticles, quantum dots, titanium dioxide nanoparticles, carbon nanomaterials (e.g., graphene), silica nanoparticles.
Uses: Engineered nanomaterials have widespread applications in electronics, cosmetics, catalysis, energy storage, drug delivery systems, and sensors.
Environmental health and safety impacts: Engineered nanomaterials may pose potential risks to human health and the environment. Their small size and unique properties can lead to increased toxicity, bioaccumulation, and potential ecological disruptions. Safe handling, proper waste management, and risk assessment are necessary to mitigate any adverse effects.
Organic Nanomaterials:
Examples: Nanocellulose, dendrimers, liposomes, organic nanoparticles (e.g., polymeric nanoparticles), nanotubes made of organic polymers.
Uses: Organic nanomaterials find applications in drug delivery, tissue engineering, electronics, flexible displays, sensors, and optoelectronics.
Environmental health and safety impacts: The environmental impact of organic nanomaterials is still under investigation. Depending on their composition and properties, they may exhibit varying levels of biocompatibility and potential toxicity. Assessments of their environmental fate, exposure routes, and potential hazards are crucial for ensuring their safe use and minimizing any adverse effects.
Inorganic Nanomaterials:
Examples: Quantum dots (e.g., cadmium selenide), metal oxide nanoparticles (e.g., titanium dioxide), silver nanoparticles, magnetic nanoparticles (e.g., iron oxide), nanoscale zeolites.
Uses: Inorganic nanomaterials are utilized in electronics, catalysis, solar cells, water treatment, imaging, and antimicrobial applications.
Environmental health and safety impacts: Inorganic nanomaterials may have environmental impacts related to their potential toxicity, persistence, and release into ecosystems. Their interactions with living organisms and ecosystems require careful assessment to ensure their safe use and minimize any negative effects.
Understanding their properties, fate, and behavior in different environments is crucial for responsible development, use, and disposal of nanomaterials.
To know more about Nanomaterials, visit
brainly.com/question/29540028
#SPJ11
how many carbon atoms are in 10.0mg of aspirin C9H8O4 molar mass
180 g mol-1
Answers
There are approximately 0.0004995 carbon atoms in 10.0 mg of aspirin.
The molar mass of aspirin (C9H8O4) is 180 g/mol. Calculate the number of carbon atoms in 10.0 mg of aspirin. The molar mass of C9H8O4 = 9 x atomic mass of C + 8 x atomic mass of H + 4 x atomic mass of O= 9 x 12.011 + 8 x 1.008 + 4 x 15.999= 180.16 g/mol.
Hence, 1 mole of aspirin weighs 180.16 g and contains 9 moles of carbon atoms (1 mole of C9H8O4 contains 9 carbon atoms). Number of moles of aspirin in 10.0 mg = 10.0 mg/180.16 g/mol= 0.0000555 mol. Number of carbon atoms in 10.0 mg of aspirin= 9 x 0.0000555= 0.0004995.
Therefore, there are approximately 0.0004995 carbon atoms in 10.0 mg of aspirin.
Learn more about the "carbon atoms" :
https://brainly.com/question/17154602
#SPJ11
The complete combustion of ethanol, to form CO2(g) and H2O(g) at constant pressure releases 726.7 kJ of heat per mole of (a) Write a balanced thermochemical equation for this reaction. a) Draw an enthalpy diagram for the reaction.
Answers
(a) First, we need to write the combustion reaction for ethanol (CH3CH2OH)
CH3CH2OH + O2 → CO2 + H2O
Then, we need to balance this equation. To do that, remember to start with C, then move on to H and finally balanced the amount of O:
CH3CH2OH + 3 O2 → 2 CO2 + 3 H2O
(b) Since this is a reaction that realeases heat (726.7 kJ /mol of ethanol), we know that is an exothermic reaction and the general scope of an enthalpy diagram would be like this:
Where, for the combustion of ethanol, the reactants would be CH3CH2OH + O2, the products would be CO2 + H2O, and ΔH would be -726.7 kJ/mol

Calculate the molality of a sucrose solution made by dissolving 37.4 grams sucrose into 94.2 grams water. The molar mass of sucrose is 342.2 g/mol. Record your answer in scientific notation using 3 significant figures
Answers
The molality of the sucrose solution is 1.16 m
Definition of molalityThe molality of a solution is defined as the mole of solute per unit kilogram (Kg) of water. Mathematically, it can be expressed as:
Molality = mole / mass (Kg) of water
How to determine the mole of sucrose Mass of sucrose = 37.4 gMolar mass of sucrose = 342.2 g/molMole of sucrose =?Mole = mass / molar mass
Mole of sucrose = 37.4 / 342.2
Mole of sucrose = 0.109 mole
How to determine the molality Mole of sucrose = 0.109 moleMass of water = 94.2 g = 94.2 / 1000 = 0.0942 KgMolality =?Molality = mole / mass (Kg) of water
Molality of sucrose = 0.109 / 0.0942
Molality of sucrose = 1.16 m
Learn more about Molality:
https://brainly.com/question/4251997
Using the table of average bond energies, estimate the energy needed to break the bonds of the reactants and the energy released when the products from for the reaction N2 + O2 –> 2NO. Note N2 has a triple bond and O2 and NO have double bonds.

Answers
The energy that is released for the breaking of bonds is 229 kJ/mol.
What is the energy released?We should be able to recall that the enthalpy of the reaction taken to be the energy that is evolved or absorbed in the reaction that is ongoing. We have to note that in the course of the reaction there would be the breaking and the making of bonds.
Now we know that;
The bond energy can be given as;
Sum energy of the broken bonds of reactants - Sum of the energy of the formed bonds
Hence;
(945 + 498) - 2(607)
1443 - 1214
= 229 kJ/mol
Learn more about enthalpy:https://brainly.com/question/13996238
#SPJ1
A cube of zinc and a cube of silver have the same volume. The mass of the zinc is 55lbs (pounds). What is the mass of the silver in kg (kilograms)?
Answers
Answer:
Explanation:
Convert 55lbs to kg
(55 lb)*(453.6 g/lb) = 24948 g or 249.48 kg
Density of zinc = 7140 kg/m^3
Find the volume occupied by 249.48 kg:
(249.48 kg)/(7140 kg/m^3) = 0.055 m^3
Density of silver = 10,490 kg/m^3
Mass of Ag in 0.055m^3:
(0.055m^3)*(10,490 kg/m^3) = 577 kg