Answers
Answer:
nuclear
Explanation:
This is because when the protons and neutrons split the energy being released is heat, also known as nuclear fission.
Answer:
The answer is Nuclear
Related Questions
Which period 2 element would the 4th ionization energy be much larger than the 3rd ionization energy?
Answers
Period 2 element would the 4th ionization energy be much larger than the 3rd ionization energy is Boron.
Boron belongs to period second. atomic no. of boron is 5 and the electronic configuration is given as :
B₅ = 1s² 2s² 3p₁
The 1st ionization energy = 800.6
the 2nd ionization energy = 2427.1
the 3rd ionization energy = 3659.7
the 4th ionization energy = 25025.8
So. we can see that the 3rd ionization energy is much lower than 4th ionization energy because after removing the 3rd electron from 2s boron will attain the stable electronic configuration of helium. now to remove electron from filled orbital it will require much larger energy the 3rd ionization energy.
To more about ionization energy here
https://brainly.com/question/11266461
#SPJ1
A coin is 3.00% Cu by mass. If the mass of the coin is 4.0561g, how many moles of Cu does it contain
Answers
Answer:
4.0561×0.03÷63.5= 0.001916 mol cu
A coin is 3.00% Cu by mass. If the mass of the coin is 4.0561g, moles of Cu does it contain 0.001916 mole of Cu.
What is mass ?It is the most fundamental characteristic of matter and one of the fundamental quantities in physics.
Mass is a term used to describe how much matter is there in a body. The kilogramme is the SI unit of mass (kg). A body's mass does not alter at any point in time.
Given mass of coin = 4.0561g , moles =? , Cu by mass= 3.00%
4.0561×0.03÷63.5= 0.001916 mole Cu.
Thus, A coin is 3.00% Cu by mass. If the mass of the coin is 4.0561g, moles of Cu does it contain 0.001916 mole of Cu.
To learn more about mass, refer to the below link:
https://brainly.com/question/13592376
# SPJ2
the earth's crust is part of which sphere?
Answers
Answer:
Lithosphere
Explanation:
The lithosphere is the solid, outer part of the Earth. The lithosphere includes the brittle upper portion of the mantle and the crust, the outermost layers of Earth's structure. It is bounded by the atmosphere above and the asthenosphere (another part of the upper mantle) below.
MARK ME AS BRAINLIEST PLS
How much ice in grams would have to melt to lower the temperature of 352 mL
of water from 15 ∘C
to 0 ∘C
? (Assume that the density of water is 1.0 g/mL
Answers
Answer:
66 grams of ice would have to melt to lower the temperature of 352 mL of water from 15 °C to 0 °C.
Explanation:
To calculate the amount of ice that would have to melt to lower the temperature of 352 mL of water from 15 °C to 0 °C, we need to use the formula:
Q = m_water * c_water * ΔT_water + m_ice * Lf
where,
Q = the amount of heat transferred,
m_water = the mass of water, c_water is the specific heat capacity of water,
ΔT_water = the change in temperature of water, m_ice = the mass of ice,
Lf = the specific latent heat of fusion of ice.
First, let's calculate the amount of heat transferred to the water:
Q = m_water * c_water * ΔT_water
Q = 352 g * 1.0 cal/(g*°C) * (15-0) °C
Q = 5,280 cal
Next, we can use the specific latent heat of fusion of ice, which is 80 cal/g, to calculate the amount of heat required to melt the ice:
Q = m_ice * Lf
Q = m_ice * 80 cal/g
m_ice = Q / Lf
m_ice = 5,280 cal / 80 cal/g
m_ice = 66 g
How many bonds are in NH4¹+?
2
3
5
4
Answers
Answer:
3 Covalent Bonds and 1 Co ordinate Bond
Explanation:
4 bonds are in NH4¹+
NH4¹+ is the ammonium ion, which consists of a central nitrogen atom (N) and four hydrogen atoms (H). Nitrogen is located in group 15 of the periodic table and has 5 valence electrons. Hydrogen, on the other hand, has 1 valence electron.
To achieve a stable electron configuration, nitrogen needs to share electrons with the hydrogen atoms. Each hydrogen atom can form a single bond with the nitrogen atom by sharing its valence electron.
In NH4¹+, all four hydrogen atoms form single bonds with the central nitrogen atom. These bonds are represented by lines connecting each hydrogen atom to the nitrogen atom.
So, NH4¹+ has 4 bonds. Each bond represents a pair of electrons shared between the nitrogen atom and a hydrogen atom. The bonding arrangement ensures that the nitrogen atom has a complete octet (eight valence electrons) and each hydrogen atom has two electrons, following the stable configuration of helium.
The "+1" charge on NH4¹+ indicates that the ion has lost one electron, resulting in a positive charge. However, the number of bonds remains the same regardless of the charge.
Therefore, the correct answer is 4 for the number of bonds in NH4¹+.
Know more about the Ammonium ion here:
https://brainly.com/question/13796846
#SPJ8
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
Given that oxygen -16 and oxygen -18 both havean atomic number of 8, how many electrons, protons and neutrons do these oxygen atoms contain
Answers
Oxygen-16: 8 protons, 8 neutrons, 8 electrons
Oxygen-18: 8 protons, 10 neutrons, 8 electrons
Explanation:Using atomic and mass numbers, we can find information about the makeup of atoms.
Protons
Every element has a unique number of protons, and every atom of the same element will have the same number of protons. This means that both oxygen atoms must have the same number of protons. The number of protons in an element is equal to its atomic number. Since oxygen has an atomic number of 8, all oxygen atoms will have 8 protons. This means that oxygen-16 and oxygen-18 have 8 protons.
Neutrons
The number of neutrons an atom of a certain element can have varies. Atoms of the same element that have different numbers of neutrons are known as isotopes. The number of neutrons can be found through the mass number. The mass number is the number that follows the dash in the name of the isotope. This number is equal to the protons plus the neutrons. So, to find the number of neutrons in an oxygen atom, subtract 8 protons from the mass number. This means oxygen-16 has 8 neutrons and oxygen-18 has 10 neutrons.
Electrons
Remember that electrons are negatively charged, protons are positively charged, and neutrons have no charge. The number of electrons in an atom determines the charge of the atom. If the number of electrons equals the number of protons, then the charge of the atom will equal 0. If there are more electrons, then the atom will be negatively charged and vice versa. Since neither of the atoms has any indication of a nonzero charge, the number of electrons must equal the number of protons. So, both oxygen atoms have 8 electrons.
Plz help me I am!!!!

Answers
a calorimeter absorbs 20,900 j of energy from the snack shown in the data below how much energy does the snack provide per gram (J/g) according to the experiment

Answers
Answer:
9.990,123578
Explanation:
0.659248-+780
Answer:
41800 j/g
Explanation:
Your welcome
Calculate ΔHrxn for the following reaction: Al2O3(s)+3CO(g)→2Al(s)+3CO2(g) Use the following reactions and given ΔH values: 2Al(s)+32O2(g)→Al2O3(s),ΔH CO(g)+12O2(g)→CO2(g),ΔH==−1675.7kJ−282.7kJ
Answers
The desired reaction is 2Al(s) + 3CO2 from Al2O3(s) + 3CO(g) (g) The reactions include 2 Al(s), 3/2 O2(g), and Al2O3(s), with H = 1675.7kJ. ————————— (1) CO(g) = CO2 + 1/2 O2(g) (g).
How is H inside a calculated?As a result, the enthalpies of a reactants and products are added together, and the result is used to compute the enthalpy of a reaction. This endothermic process generates and absorbs environmental heat if H is positive. This reaction is exothermic so emits heat into the environment if H is negative.
What is the H heat?A negative H indicates that heat is transferred from the a system towards its surroundings, whereas a positive H indicates that heat is transferred from the surroundings into the system. An enthalpy of reaction (Hrxn) for a chemical reaction is the difference of enthalpy between the products and reactants; Hrxn is measured in kilojoules per mole.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

How many moles of phosphoric acid would be needed to produce 15 grams of water?
Answers
Moles of phosphoric acid would be needed : 0.833
Further explanationGiven
15 grams of water
Required
moles of phosphoric acid
Solution
Reaction(decomposition) :
H3PO4 -> H2O + HPO3
mol water (H2O :
= mass : MW
= 15 g : 18 g/mol
= 0.833
From the equation, mol ratio H3PO4 = mol H2O = 1 : 1, so mol H3PO4 = 0.833
The vertical axis of a graph shows the
Answers
Answer:
The horizontal axis represents years and the vertical axis represents units of sales. The graph presents both the increase and decline in sales for both products, as sales fluctuated during the ten-year period.
Explanation:
How many grams of O2 are required to produce 358.5 g of ZnO
Answers
Answer:35.24 grams of are required to produce the given amount of Zinc oxide.
What information does an acid or base equilibrium constant give?
Answers
The value of the equilibrium constant shows the relative amounts or concentrations of the reactants and products.
Will mark brainliest
Calculate the specific heat of a substance given that 512 joules of heat is required to raise the temperature of 255.0 g of the substance by 15.0 ºC.
Answers
Answer:
\(c=0.133\ J/g^{\circ}C\)
Explanation:
Given that,
Heat required, Q = 512 J
Mass of the substance, m = 255 g
The change in temperature, \(\Delta T=15^{\circ} C\)
Let c be the specific heat of the substance. We know that the heat required to raise the temperature is given by :
\(Q=mc\Delta T\)
Where
c is the specific heat of a substance
So,
\(c=\dfrac{Q}{m\Delta T}\\\\=\dfrac{512}{255\times 15}\\\\c=0.133\ J/g^{\circ}C\)
So, the specific heat of the substance is equal to \(0.133\ J/g^{\circ}C\).
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
Bonding with intermolecular forces:
1.) is CH3F a hydrogen bonding? yes or no?
2.) is CH3F dipole-dipole interactions? Yes or no?
3.) is CH3F a london dispersion forces? Yes or no?
Answers
Explanation:
1.) is CH3F a hydrogen bonding? yes or no? = No, CH3F is not a hydrogen bonding beacuse the molecule lacks hydrogen atoms bonded to nitrogen, oxygen, or fluorine; ruling out hydrogen bonding.2.) is CH3F dipole-dipole interactions? Yes or no? = Yes, CH3F is a dipole-dipole interactions beacuse in it's molecule, there are no metal atoms to form ionic bonds3.) is CH3F a london dispersion forces? Yes or no?= Yes, CH3F is a london dispersion forces because in it's molecule, there are no metal atoms to form ionic bonds .The intermolecular forces present in the bonding of CH₃F are dipole-dipole interactions and London dispersion forces.
1.) No, CH₃F does not exhibit hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom such as fluorine, oxygen, or nitrogen. In CH₃F, the hydrogen atom is bonded to carbon, which is not highly electronegative.
2.) Yes, CH₃F exhibits dipole-dipole interactions. Dipole-dipole interactions occur between molecules that have permanent dipoles due to the electronegativity difference between the atoms. In CH₃F, the fluorine atom is more electronegative than the carbon and hydrogen atoms, resulting in a polar molecule with a permanent dipole moment.
3.) Yes, CH₃F exhibits London dispersion forces. London's dispersion forces, also known as Van der Waals forces, are present in all molecules and arise from temporary fluctuations in electron distribution. Although CH₃F has dipole-dipole interactions, it also experiences London dispersion forces due to the temporary shifts in electron density.
Hence, the bonding in CH₃F was explained above.
Learn more about intermolecular forces here:
https://brainly.com/question/31797315
#SPJ 6
The chemical formula for baking soda is NaHCO3. A 168.02-g sample of baking soda contains 45.98 g of sodium, 2.02 g of hydrogen, 24.02 g of carbon, and 96 g of oxygen. What is the mass percentage of each element in baking soda?
Answers
The mass percentage of each element in baking soda is:
For sodium, it is 27.36%.For hydrogen, it is 1.2%.For carbon, it is 14.3%.For oxygen, it is 57.14%The calculation of mass percentage for each element is as follows:
For sodium \(= \frac{45.98}{168.02} \times 100\%\) = 27.36%For hydrogen \(= \frac{2.02}{168.02} \times 100\%\) = 1.2%.For carbon \(= \frac{24.02}{168.02} \times 100\%\) = 14.3%For oxygen \(= \frac{96}{168.02} \times 100\%\) = 57.14%In this way, the mass percentage should be calculated by dividing each one from the baking soda sample.
Learn more: brainly.com/question/13145357
Answer:
The mass percentages of sodium, hydrogen, carbon, and oxygen are 27.37%, 1.20%, 14.30%, and 57%, respectively.
Explanation:
The mass percentage (%mass) of an element present in a compound is calculated using the following expression:
\(\rm \%mass=\dfrac{mass\;of\;the\;element}{mass\;of\;the\;compound}\times 100\%\)
According to the given problem, the mass of \(\rm NaHCO_3\) is 168.02 g.
The mass of sodium in the given sample is 45.98 g.
So, the %mass of sodium in the given sample is calculated as shown below:
\(\rm \%mass\;of\;sodium=\dfrac{45.98\;g}{168.02\;g}\times 100\%=27.37\%\)
Similarly, the mass percentages of hydrogen, carbon, and oxygen are calculated as shown below:
\(\rm \%mass\;of\;hydrogen=\dfrac{2.02\;g}{168.02\;g}\times 100\%=1.20\%\)
\(\rm \%mass\;of\;carbon=\dfrac{24.02\;g}{168.02\;g}\times 100\%=14.30\%\)
\(\rm \%mass\;of\;oxygen=\dfrac{96\;g}{168.02\;g}\times 100\%=57\%\)
Hence, the mass percentages of sodium, hydrogen, carbon, and oxygen are 27.37%, 1.20%, 14.30%, and 57%, respectively.
Learn more about mass percentage here:
https://brainly.com/question/12073721
Which does NOT lead to elevated levels of carbon in the atmosphere?
a
increased burning of fossil fuels
b
industrial revolution
c
planting more trees
d
deforestation
Answers
how to separate bean from mixture without picking them out one by one
Answers
For example Chromatography involves solvent separation on a solid medium and distillation takes advantage of differences in boiling points
Answer the following question attached!!
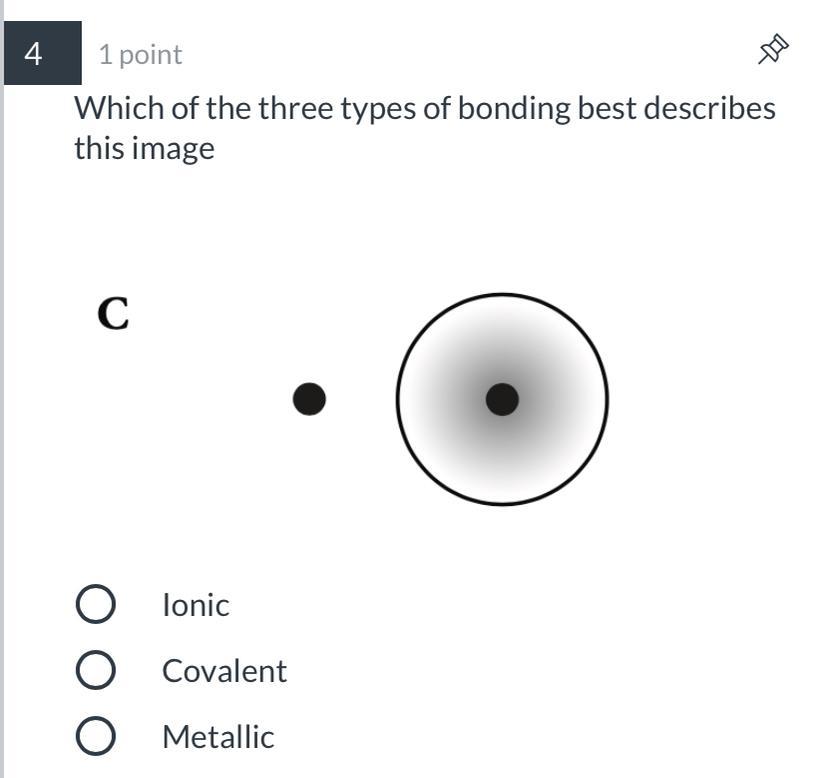
Answers
Answer:
I think It is Covalent. Not 100% sure but like 90% sure.
The reaction of a Grignard reagent with an aldehyde followed by dilute acid gives a (n) 20) A) secondary alcohol. C) ester B) primary alcohol. D) tertiary alcohol
Answers
The reaction of a Grignard reagent with an aldehyde followed by dilute acid gives a secondary alcohol.
Given reaction is followed:
\(CH_{3}CHO \rightarrow CH_{3}COHHph\) in presence of phMgBr, ether and \(H_{3}O^{+}\)Aldehydes or ketones' carbonyl group, C=O, reacts with organolithium or Grignard reagents to produce alcohols. The type of alcohol produced depends on the carbonyl's substitutes.
A secondary alcohol is a substance that contains a saturated carbon atom with two additional carbon atoms linked to it and a hydroxy group, or OH.
A chemical molecule with the generic formula RMgX, where X is a halogen and R is an organic group, typically an alkyl or aryl, is known as a Grignard reagent or Grignard compound.
Learn more about Grignard reagent here:
https://brainly.com/question/16040954
#SPJ4
Imagine that you mix 25 g of water at 25 ºC with 25 g of water at 65 ºC. Predict the final temperature of the sample.
Answers
The final temperature of the mixture given that 25 g of water at 25 °C is mixed with 25 g of water at 65 °C, is 45 °C
How do i determine the final temperature of the mixture?The final temperature of the mixture can be obtained by calculating the equilibrium temperature of the mixture. This is shown below:
Mass of cold water (M) = 25 gTemperature of cold water (T) = 25 °CMass of warm water (Mᵥᵥ) = 25 gTemperature of warm water (Tᵥᵥ) = 65 °CEquilibrium temperature (Tₑ) =?Heat loss by warm water = Heat gain by cold water
MᵥᵥC(Tᵥᵥ - Tₑ) = MC(Tₑ - T)
Cancel out C
Mᵥᵥ(Tᵥᵥ - Tₑ) = M(Tₑ - T)
25× (65 - Tₑ) = 25 × (Tₑ - 25)
Cancel out 25
65 - Tₑ = Tₑ - 25
Collect like terms
65 + 25 = Tₑ + Tₑ
90 = 2Tₑ
Divide both side by 2
Tₑ = 90 / 2
Tₑ = 45 °C
Thus, we can conclude that the final temperature the mixture is 45 °C
Learn more about temperature:
https://brainly.com/question/14281142
#SPJ1
A 78.0 mL portion of a 1.70 M solution is diluted to a total volume of 218 mL. A 109 mL portion of that solution is diluted by adding 115 mL of water. What is the final concentration? Assume the volumes are additive.
Answers
Taking into account the definition of dilution, the final concentration after two dilutions is 0.296 M.
Definition of dilutionDilution is the reduction in concentration of a chemical substance in a solution. This is accomplished by adding more solvent to the solution at the same amount of solute.
The amount of solute does not change, but as more solvent is added, the concentration of the solute decreases, as the volume of the solution increases.
A dilution is mathematically expressed as:
Ci×Vi = Cf×Vf
where
Ci: initial concentrationVi: initial volumeCf: final concentrationVf: final volumeFinal concentrationIn this case, you have a first dilution where you know:
Ci= 1.70 M Vi= 78 mLCf= ?Vf= 218 mLReplacing in the definition of dilution:
1.70 M× 78 mL= Cf× 218 mL
Solving:
(1.70 M× 78 mL)÷ 218 mL= Cf
0.608 M= Cf
A 109 mL portion of that solution is diluted by adding 115 mL of water. So, you have a second dilution where you know:
Ci= 0.608 M Vi= 109 mLCf= ?Vf= Vi + Added volume of water= 109 mL + 115 mL= 224 mLReplacing in the definition of dilution:
0.608 M× 109 mL= Cf× 224 mL
Solving:
(0.608 M× 109 mL)÷ 224 mL= Cf
0.296 M= Cf
In summary, the final concentration is 0.296 M.
Learn more about dilution:
brainly.com/question/6692004
#SPJ1
How much energy would it take to heat a section of the cooper tubing that weighs about 650.0g, from 15.83C to 24.11C
Answers
Energy would it take to heat a section of the copper tubing that weighs about 650.0g, from 15.83°C to 24.11°C is 2.072 KJ.
The formula heat energy is given by :
q = mcΔT
given that :
m = 650.0 g
specific heat of copper c = 0.385 J/g °C
T1 = 15.83°C
T2 = 24.11°C
ΔT = T2 - T1 = 24.11°C - 15.83°C = 8.28 °C
putting all the values in the formula :
q = mcΔT
q = 650.0 g × 0.385 J/g °C × 8.28 °C
q = 2072.2 J = 2.072 KJ
Thus, Energy would it take to heat a section of the copper tubing that weighs about 650.0g, from 15.83°C to 24.11°C is 2.072 KJ.
To learn more about heat energy here
https://brainly.com/question/15045931
#SPJ1
Approximately how many moles of Al3+ are reduced when 0.1 faraday of charge passes through a cell during the production of AI? (Note: Assume there is excess AP+ available and that Al3+ is reduced to Al metal only.) A. 0.033 mol B. 0.050 mol C. 0.067 mol D. 0.10 mol
Answers
A faraday is equal to one mole of electric charge. Because each aluminum ion gains 3 electrons, 0.1 faraday of charge will reduce 0.1/3 moles of aluminum, or 0.033 moles of aluminum.
What is meant by aluminum ?
Aluminum was formally adopted by the American Chemical Society (ACS) in 1925, but the International Union of Pure and Applied Chemistry (IUPAC) only did so in 1990.Including thus we arrive at the present, where aluminum is used everywhere else and by English-speaking people in North America.Aluminium is a light, silvery-white metal. It is bendable and soft. Numerous items, such as cans, foil, culinary utensils, window frames, beer kegs, and airplane components, are made of aluminum.Chemical element aluminum (Al), also written aluminum, belongs to the major Group 13 (IIIa, or boron group) of the periodic table. It is a light silvery white metal. The most prevalent nonferrous metal and the most plentiful metallic element in the crust of the Earth is aluminum.To learn more about aluminum refer to
https://brainly.com/question/246454
#SPJ4
LAST ATTEMPT IM MARKING AS BRAINLIEST!! (What is the name of the COVALENT compound below ? )

Answers
Sorry if it’s wrong i haven’t done that in a whole
Answer:
its number 3 trust me
Explanation:
CAN SOMEONE HELP ME PLZ AND THANKS WILL MARK U AS BRAINLIEST

Answers
Explanation:
2. \(2C_2H_6 + 7O_2 \rightarrow 4CO_2 + 6H_2O\)
First, we need to find the number of moles of \(CO_2\) at 300K and 1.5 atm using the ideal gas law:
\(n= \dfrac{PV}{RT}= \dfrac{(1.5\:\text {atm})(33\:L)}{(0.082\:\text{L-atm/mol-K})(300K)}\)
\(=2.0\:\text{mol}\:CO_2\)
Now use the molar ratios to find the number of moles of ethane to produce this much \(CO_2\).
\(2.0\:\text{mol}\:CO_2 \times \left(\dfrac{2\:\text{mol}\:C_2H_6}{4\:\text{mol}\:CO_2}\right)\)
\(=1.0\:\text{mol}\:C_2H_6\)
Finally, convert this amount to grams using its molar mass:
\(1.0\:\text {mol}\:C_2H_6 \times \left(\dfrac{30.07\:\text g\:C_2H_6}{1\:\text{mol}\:C_2H_6} \right)\)
\(=30.1\:g\:C_2H_6\)
3. \(3Zn + 2H_3PO_4 \rightarrow 3H_2 + Zn_3(PO_4)_2\)
Convert 75 g Zn into moles:
\(75\:\text g\:Zn \times \left(\dfrac{65.38\:\text g\:Zn}{1\:\text{mol}\:Zn}\right)=1.1\:\text{mol}\:Zn\)
Then use the molar ratios to find the amount of H2 produced.
\(1.1\:\text{mol}\:Zn \times \left(\dfrac{3\:\text{mol}\:H_2}{3\:\text{mol}\:Zn}\right)=1.1\:\text{mol}\:H_2\)
Now use the ideal gas law \(PV=nRT\) to find the volume of H2 produced at 23°C and 4 atm:
\(V= \dfrac{nRT}{P}= \dfrac{(1.1\:\text{mol}\:H_2)(0.082\:\text{L-atm/mol-K})(296K)}{4\:\text{atm}}\)
\(=8.9\:\text L\:H_2\)
Substances that are composed of a network of atoms held together by covalent bonds are called
O A. ionic lattices
B. giant covalent structures
C. allotropes
D. diatomic networks
Answers
Answer:
giant covalent structures
Explanation:
Covalent solids are made up of atoms joined to one another by covalent bonds to form a giant lattice. The covalent bonds are very strong, this makes the giant covalent solids to be hard and possess high melting points. Covalent solids are nonconductors of electricity due to the absence of free electrons in the structure.
Diamond, graphite and boron nitride are all examples of giant covalent structures.