Both chairs are the same size and have the same number of molecules. The diagram above shows the chairs before they touch.
How does the temperature of the bottom chair compare with the temperature of the top chair before the chairs touch? What will happen after the chairs have been touching for a while?
Answers
Before the chairs touch, the temperature of the bottom chair is lower than the temperature of the top chair, this is because the molecules in the bottom chair are in contact with a cooler surface.
After the chairs have been touching for a while, the heat will begin to transfer from the top chair to the bottom chair through a process called conduction. This will continue until the temperature of the two chairs equalizes, at which point there will be no more net heat transfer between them.
The final temperature of both chairs will be somewhere between the initial temperatures of the two chairs, and will depend on factors such as the thermal conductivity of the material, the size of the chairs, and the duration of the contact.
To know more about the Molecules, here
https://brainly.com/question/2114821
#SPJ1
Related Questions
calculate the volume of carbon dioxide of room temperature and pressure obtained from 30 grams of glucose
Answers
Answer:
A
Explanation:
Guys I really need to you answer this question for me pleaseeeee. Describe one situation in which forces are created.
Answers
The application of force in the direction of the motion of an object. The second scenario involves applying force to a moving item that is traveling in the opposite direction.
What is force ?A force is an influence that has the power to alter an object's motion. An object with mass can change its velocity, or accelerate, as a result of a force. A force has both a direction and a magnitude.
Force is used to describe a body's tendency to modify or change its state as a result of an external cause. When force is applied, the body can also alter its size, shape, and direction.
A push or pull that an object experiences as a result of interacting with another item is known as a force. Every time two items interact, a force is exerted on each of the objects. The force is no longer felt by the two objects when the interaction ends.
Thus, Force applied to an item in motion that originates in any direction constitutes the third situation where force is created.
To learn more about force, follow the link;
https://brainly.com/question/13191643
#SPJ1
What is The Magnus Effect/Force? Explain.
Answers
Answer:
dk
Explanation:
The solute in the above clip is SucroseSucrose (C12H22011) is an example of aIn Sucrose (C12H22011), the least electronegative element isas it exists in a fixed ratio.In Sucrose (C12H22011), unlike C and O, H likes to achieve its stability by following a‡ et rule.Once dissolved in water, sucrose will dissociate into a cation and anion-True or False


Answers
The best way of characterizing the sucralose would be to say it is a molecule, but there is no such option. So, the closest options is "Compound", because it is composed by more than one element.
So, first blank is "Compound".
The elements on it are H, C and O. From an electronegativity table, we can see that the electronegativities are approximately:
H: 2.1
C: 2.5
O: 3.5
So, the least electronegative is H.
Second blank is "hydrogen".
The normal rule to follow is the octet rule, so we complete the shell by getting 8 electron in it.
But, the hydrogen has only 1 electron and it is on the first shell, so it can't complete the octet rule like normal, it follows the duet rule, that is, it completes 2 electrons.
Third blank is "Du".
The Sucralose is composed by nonmetals and hydrogen only, so they don't bond by ionic bonds, they form covalent bonds. Thus, they don't dissociate into ions.
Fourth blank is "False".
A bond is considered polar if the electronegativity of their elements are dfferent enough. When bonding C-C, both have the same electronegativity, so they are not polar, that is, they are non polar covalent bonds.
Fifth blank is "Non polar covalent".
O-H, on the other hand, have a very different electronegativities, one is 2.1 and the other is 3.5, so the bond will be polar.
Sixth blank is "Polar covalent".
Between the solid, liquid and gas phases, the gas is when the molecules are the most apart and solid is when the molecules are the closest. Since it is solid, the particules will be closer.
Seventh blank is "Closer".
.
Which of these properties is the best one to use for indentification of an element
Answers
Answer:
you need to state the options
Solar and wind energy are both intermittent resources that cannot be relied upon for a constant stream of energy production. Explain why developing better ways to store energy is an important part of making these energy sources more practical to use.
Answers
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers.
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers. Energy storage's inherent ability to offer backup power in the event of grid failure is a feature that both residential consumers and commercial owners find highly desirable.
To know more about energy, here:
https://brainly.com/question/1932868
#SPJ1
please I'm taking the unit test I need help
Which process is defined as breaking down rocks into smaller pieces without changing the rocks' compositions?
erosion
oxidation
chemical weathering
mechanical weathering
Answers
Answer:
Mechnical weathering
Explanation:
Answer:
D edge 2021 ;)
Explanation:
I need to know the answer to this science problem . Please and thank you

Answers
Step 1 - Understanding the types of heat transfer
There are three types of heat transfer: conduction, convection and irradiation. Let's see how each one works:
a) Conduction: is when a very hot substance enters in contact with a cooler substance. The atoms in the hotter substance are moving with a greater velocity and will therefore collide with the atoms of the cooler substance.
b) Convection: it happens especially in liquids and air (fluids). The liquid is warmed up at the bottom first, via conduction. The heated bottom thus changes its density: it becomes less dense, and go up. This process repeats itself several times, warming the whole liquid.
c) Irradiation: when something is put near a heat source, but do not touch it directly. In this case, there's no direct atom collision, but indirect: the rapid atoms of the heat source collide with atoms in the air which then collide with the atoms in the object that is being heated.
Step 2 - Discovering the type of heat transfer in each scenario
In the first scenario, the water at the bottom is in direct contact with the pan. Therefore, water molecules will directly collide with "pan" molecules (probably aluminum atoms or other materials). But there's also convection. The water is not heated only by conduction.
In the second scenario, the hands are near the Bunsen burner, but not directly touching it. What is happening here is then heat irradiation, not conduction.
In the third scenario, the hand is touching the object (the handle of the pan). Even though handles are made of material that poorly conduct heat, it will increase its temperature, at least a little bit. When we touch it, we can feel it is hotter than before. In this case, only conduction is involved (from the handle to our hand). In this case atoms are directly colliding and this is the only source of heat.
Finally, in the last scenario, we also have a case of irradiation: the pan with water is near the heat source, but not directly touching it.
Step 3 - How to set an experiment
In science in general, anytime we want to investigate some effect it is good manners to investigate a system controling all other effects. We want to investigate a variable at a time.
Therefore, while both scenario 1 and 3 involve conduction, scenario 1 also involves convection, which could be a problem to a experiment intending to study conduction only.
The best experiment would be then scenario 3, hands touchind the handle of pan siting in a Bunsen burner.
How many moles of Sb,03 will be formed when you have 20.0 moles of oxygen gases?
Answers
20.0 moles of oxygen react with Antimony to form 13.3 moles of Antimony (III) Oxide. We want to calculate how many moles of Antimony (III) Oxide will be formed from 20.0 moles of oxygen. This is a stoichiometry problem.
What is stoichiometry?The link between the proportional amounts of components participating in a reaction or generating a compound is known as stoichiometry, and it is often expressed as a ratio of whole integers.
Assuming a balanced chemical equation, the stoichiometric ratio between Antimony (III) Oxide and oxygen can be used to determine the number of moles of Antimony (III) Oxide formed.
For example, the balanced equation for the reaction of Antimony with O2 to form Antimony (III) Oxide is:
4 Antimony + 3 O2 → 2 Antimony (III) Oxide
From this equation, it can be seen that 3 moles of oxygen react with 2 moles of Antimony (III) Oxide . Therefore, if there are 20.0 moles of O2, then the number of moles of Antimony (III) Oxide formed would be:
20.0 moles oxygen × (2 moles Antimony (III) Oxide / 3 moles oxygen) = 13.3 moles Antimony (III) Oxide.
To know more about moles visit:-
https://brainly.com/question/26416088
#SPJ1
can somone help with this please
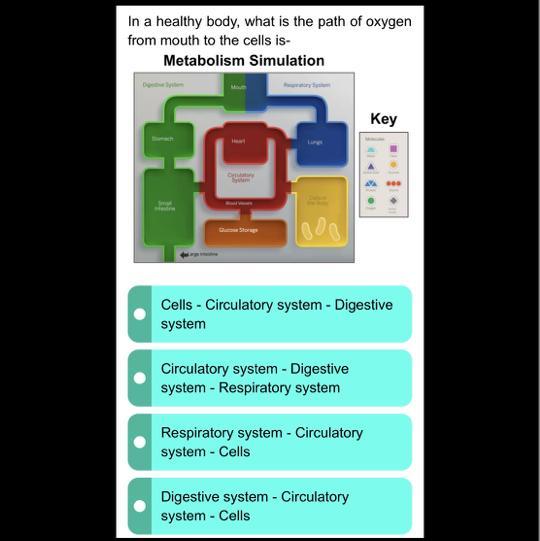
Answers
Answer:
digestive system-circularory systems -cells
What does a branching tree diagram show?
A. The evolution in the size of organisms
B. The order in which specific traits may have evolved
C. The animal population in a specific time period
D. The rate of extinction of specific traits in living organisms
Answers
Answer:
B. The order in which specific traits may have evolved
Explanation:
The basic unit of structure and function of living things is the .
nucleus
cell
tissue
membrane
Answers
Answer &
Explanation:
eukaryotic: Having complex cells in which the genetic material is contained within membrane-bound nuclei. cell: The basic unit of a living organism, consisting of a quantity of protoplasm surrounded by a cell membrane, which is able to synthesize proteins and replicate itself.
therefore it is cell
Your car breaks down. As your friends help
you puch it, it begins to move and speed
up.
Answers
try stopping putting the car in park before fixing
A flask containing 9.20 mL of a liquid weighs 184.0 g with the liquid in the flask and 176.3 g when empty. Calculate the density of the liquid in g/ mL to the correct number of significant digits.
Answers
Density is the ratio between the mass of a material and its volume (d = m/V) at a given temperature and pressure.
So first we need to find the mass of the material. We can do it calculating the mass of the flask with the liquid minus the mass of the flask without the liquid.
mass of the flask with the liquid = 184.0 g
mass of the flask without the liquid = 176.3 g
mass of the material = 184.0 - 176.3 = 7.7 g
Now we use the following equation: d = m/V
where:
d = density
m = mass = 7.7 g
V = 9.20 mL
d = 7.7/9.20
d = 0.84 g/mL
Answer: d = 0.84 g/mL
Pls help me out ASAP
A sample of nitrogen dioxide, NO, is held at a temperature of 1195 K in a container with a volume of 25 liters and a pressure of 560 atm. How many grams of NO, are present in the container? Round your answer to two significant figures.
A) 5300g
B) 3300g
C)6600g
D)4900g
Answers
Aside from direct harm, what secondary issue do invasive species present?
They may be able to eat all kinds of native organisms.
They may not have any predators.
They might damage the habitat that other organisms rely on.
They might introduce other organisms like viruses and bacteria.
Answers
Answer:
They may not have any predators.
Explanation:
How many grams of oxygen gas will be produced when 2.50 moles of potassium chlorate is decomposed?
Answers
Answer:
\(m_{O_2}=120gO_2\)
Explanation:
Hello!
In this case, since the decomposition of potassium chlorate is:
\(2KClO_3\rightarrow 2KCl+3O_2\)
We can see a 2:3 mole ratio between potassium chlorate and oxygen (molar mass 32.0 g/mol), thus, via stoichiometry, we compute the mass of oxygen that are produced by the decomposition of 2.50 moles of this reactant:
\(m_{O_2}=2.50molKClO_3*\frac{3molO_2}{2molKClO_3} *\frac{32.0gO_2}{1molO_2}\\\\m_{O_2}=120gO_2\)
Best regards!
Lab Report Guide
2. What procedure did you use to complete the lab?
Outline the steps of the procedure in full sentences.

Answers
Answer:
Explanation:
To be lab prepared one must follow these procedures-
1. One should have the knowledge of lab assignments to make the lab experiment easier.
2. To be aware about safety equipment and their uses in lab, like- the location of fire extinguisher in lab.
3. To know the steps of experiments to be performed
4. To fill notebook of lab with information regarding the experiment
5. One should review the data sheets of chemicals material safety.
6. To put on all the necessary dressings to perform experiment.
7. To have complete understanding about the experiment disposals.
Are these both considered lewis structureseven though one of them has lines


Answers
Yes, both of them are considered lewis structures
ExplanationsLewis structures are ways in which the valence electron of a compound are arranged around its elements using dots. It represents and shows how electrons are arranged around individual atoms.
In lewis structure, electrons are represented as dots and if it is a bonding electron, it can be represented as lines. Since the elements in the compound given are both non-metals, they are covalently bonded. This shows the reason why one of the lewis structures contains lines.
1.What happens when non-metals react with oxygen? (1 Point)
a) Metal oxides are formed.
b) Basic oxides are formed.
c) Acidic oxides are formed
Answers
Answer:
c)
Explanation:
acidic oxides are formed
metal oxides and basic oxides are basically the same.
How many moles of hydroxide ions (OH-) are found in 24.42 mL?
C. How many moles of sulfuric acid, H2SO4, are neutralized by 24.42 mL of 0.236 M NaOH(aq)?
Answers
A solution of 0.236 M NaOH containing 0.00576 molecules of hydroxide ions (OH-) is contained in 24.42 mL. Thus, 0.00288 molecules of sulfuric acid can be neutralised by 24.42 mL of 0.236 M NaOH. (H2SO4).
What kind of chemistry does Na2SO4 h2o H2SO4 NaOH represent?A neutralisation process between sodium oxide and sulfuric acid is represented by the equation above. (base and acid). An acid and a base react to create salt (Sodium Sulphate) and water as a byproduct.
We make the assumption that the hydroxide ions are present in a solution of sodium hydroxide in order to determine the number of moles of hydroxide ions (OH-) present in 24.42 mL. (NaOH)
First, we need to convert the volume of the solution from milliliters (mL) to liters (L):
24.42 mL = 0.02442 L
Next, we can use the concentration of NaOH to calculate the number of moles of OH-:
0.236 mol/L (concentration of NaOH) x 0.02442 L
= 0.00576 moles of OH-
To know more about hydroxide visit:-
https://brainly.com/question/21904397
#SPJ9
A candle snuffer is a long handle with a small bell-shaped piece of metal on the end of it that is used to snuff out burning candles. When this device is used, what is the limiting reactant in the combustion reaction happening at the candle wick?A.) oxygenB.) candle snufferC.) waterD.) candle wax
Answers
When we use the candle snuffer, it limits the amount of oxygen around the candle. The candle needs oxygen to stay lit, so the answer is the oxygen is the limiting reactant.
Answer: Oxygen
Check Your Learning a. To three decimal places, what is the volume of a cube (cm 3) with an edge length of 0.843 cm? b. If the cube in part (a) is copper and has a mass of 5.34 g, what is the density of copper to two decimal places?
Answers
1. The volume of the cube (cm³) is 0.599 cm³
2. The density of the copper to two decimal place is 8.91 g/cm³
What is density?The density of a substance is simply defined as the mass of the subtance per unit volume of the substance. Mathematically, it can be expressed as
Density = mass / volume
1. How to determine the volume of the cubeLength (L) = 0.843 cmVolume (V) =?V = L³
V = 0.843³
V = 0.599 cm³
How to determine the densityMass = 5.34 gVolume = 0.599 cm³Density =?Density = mass / volume
Density = 5.34 / 0.599
Density = 8.91 g/cm³
Learn more about density:
https://brainly.com/question/952755
#SPJ1
How are ocean waves formed? gravity energy transfer conduction radiation

Answers
Answer:
I apologize that I'm late and all But your answer is B. Energy Transfer.
Explanation:
The waves transfer energy to the sand for example.
How are scientific questions answered? OA. Through feelings and guesses
B. Through likes and dislikes
C. Through beliefs and opinions
D. Through measuring and observing
Answers
convert 9.32x 10 23ª atoms of au to moles of au
Answers
Answer:
\(\huge\boxed{\sf no.\ of\ moles = 1.55\ moles }\)
Explanation:
Given:
Number of atoms = \(9.32 \times 10^{23}\) atoms
Avogadro's Number = \(6.023 \times 10^{23}\) atom / mol
Required:
Moles = ?
Formula:
\(\displaystyle No.\ of\ moles = \frac{no. \ of \ atoms }{avogadro's \ no.}\)
Solution:
\(\displaystyle no. \ of \ moles = \frac{9.32\times 10^{23}}{6.023 \times 10^{23}}\)
no. of moles = 1.55 moles
\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807A 345.21 g sample of a compound was found to contain 200.50 g of carbon and
144.71 g of hydrogen. What is the percentage composition of the compound?
Answers
Answer:
See below
Explanation:
carbon % = 200.5 / 345.21 * 100% = 58.1 %
hydrogen% = 144.71 / 345.21 *100% = 41.9 %
I need help calculating the error % in molar mass
Answers
Error % = |(experimental - actual) / actual| x 100%
For example, let's say the actual molar mass of a compound is 100 g/mol, and the experimental molar mass determined in the lab is 95 g/mol. The error percentage would be:
Error % = |(95 - 100) / 100| x 100%
Error % = |-0.05| x 100%
Error % = 5%
Therefore, the error percentage in molar mass is 5%
Helpp me plsssss!!!!!!!!!!!!!

Answers
Answer: the bottom one is wave the one above is electromagnetic and the one above is transverse
Explanation:
Examine the map of the United States. Tornadoes are most common in the Midwest and Great Plains states. According to the map, what causes the prevalence of tornadoes in these regions? A) Falling air currents form from the mixing of falling warm, moist air and the rising of cold air. B) Spinning air currents form from the mixing of rising warm, moist air and the falling of cold air. C) Spinning air currents form when cold, dry air becomes saturated with moisture and becomes unstable. Eliminate D) Rising air currents form from the deposition of cold air at the surface and moist air at the top of the troposphere.
Answers
Answer:
the answer is B-Spinning air currents form from the mixing of rising warm, moist air and the falling of cold air.
Explanation: