Bismuth oxide reacts with carbon to form bismuth metal:
Bi₂O₃(s) + 3C(s) → 2Bi(s) + 3CO(g)
When 283 g of Bi₂O₃ reacts with excess carbon, how many
(b) moles of Bi form?
Answers
(a) 12.16 mol Bi
(b) 51.03. g CO
Elaborating :
Step 1: Convert 283 g to moles
Bi Molar Mass - 208.98 g/mol × 2 = 417.98 g/mol
O Molar Mass - 16.00 g/mol × 3 = 48.00 g/mol
283 g Bi₂O₃ ÷ 465.98 g/mol = 0.6073 mol Bi₂O₃
Step 2: Find the conversion from Bi₂O₃ to Bi
1 mole of Bi₂O₃ equals 2 moles of Bi
Step 3: Use Dimensional Analysis
0.6073\(\frac{2 mol Bi}{1 mol Bi_{2}O_{3} }\) mol · = 12.1690 mol Bi
12.1690 mol Bi = 12.16 mol Bi
Step 4: Find the conversion from Bi₂O₃ to CO
1 mole of Bi₂O₃ equals 3 moles of CO
Step 5: Use Dimensional Analysis
0.6073 mol ·\(\frac{3 molCO}{1 mol Bi_{2}O_{3} }\) = 1.8219 mol CO
Step 6: Find molar mass of CO and convert moles to grams
C - 12.01 g/mol
O - 16.00 g/mol
1.8219 mol CO · 28.01 g/mol = 51.031 g CO
51.031 g CO
What is it called when two metals react?
This occurs when two different metals are in contact in a corrosive or conductive environment and the current flow changes. When two dissimilar metals are involved, the reaction is called galvanic corrosion
Why do metals react with each other?
Metal ions are positively charged as they lose negative electrons. Some metals give up their electrons more readily than others and are, therefore, more reactive. Metals can be ranked according to their level of reactivity to form the metal reactivity series.
Learn more about metal reaction :
brainly.com/question/17469010
#SPJ4
Related Questions
one practical radioactive system used to date lava flows involves: group of answer choices the gas argon-40, which decays to the gas potassium-40. the gas argon-40, which decays to solid potassium-40. the solid potassium-40, which decays to solid argon-40. the solid potassium-40, which decays to the gas argon-40. the solid potassium-40, which decays to the solid moosemossium-41.
Answers
The practical radioactive system used to date lava flows involves the gas argon-40, which decays to the gas potassium-40. Option A is correct.
This is known as the potassium-argon dating method, which is commonly used to date volcanic rocks and minerals. Potassium-40 decays into argon-40 with a half-life of 1.25 billion years, and the ratio of argon-40 to potassium-40 can be used to determine the age of the sample.
A radioactive system refers to a group of radioactive atoms that decay over time according to a specific decay scheme. The decay of radioactive isotopes occurs at a constant rate, known as the half-life, which is the time it takes for half of the original atoms to decay.
Radioactive systems are used in various applications, including dating geological materials, medical imaging, and nuclear energy production. Different radioactive isotopes have different half-lives and decay schemes, which determine their usefulness for different applications.
Hence, A. is the correct option.
To know more about radioactive here
https://brainly.com/question/1770619
#SPJ4
--The given question is incomplete, the complete question is
"One practical radioactive system used to date lava flows involves: group of answer choices A) the gas argon-40, which decays to the gas potassium-40. B) the gas argon-40, which decays to solid potassium-40. C) the solid potassium-40, which decays to solid argon-40. D) the solid potassium-40, which decays to the gas argon-40. E) the solid potassium-40, which decays to the solid moosemossium-41."--
What type of decay does Am-241 undergo to become Np-237?
a. Alpha decay
c. Positron emission
b. Beta decay
d. Electron capture
Answers
The type of decay that Am-241 undergoes to become Np-237 is alpha decay. The correct option is a.
What is alpha decay?Alpha decay is a type of decay or transformation in which a nucleus emits an alpha particle and change into different atomic nuclei. The atomic number reduces by 2 and the mass is reduced by 4.
Am-241 is an isotope of the chemical element americium. It is a radioactive element and its radioactive decay period is 432.2 years. It is used in the testing of machinery.
When the nucleus of Am changes or emits a radioactive particle. It converts into Np-237. The process is called alpha decay. The atomic mass is reduced by four, and the 241 converts into 237, which become neptunium.
Thus, the correct option is a. Alpha decay.
To learn more about alpha decay, refer to the link:
https://brainly.com/question/27870937
#SPJ2
How many total atoms does each element have?
Plz help!

Answers
Answer:
14 Calcium (4x3+2)
2 Phosphurus (2)
9 Oxygen (4x2+1)
The reaction between ethyl bromide and sodium cyanide in acetone is most likely to be?
Answers
The reaction between ethyl bromide and sodium cyanide in acetone is most likely an SN2 reaction that is characterized by a strong nucleophile and polar aprotic acetone.
What is the SN2 mechanism?The SN2 mechanism is the reaction that is generally seen in organic compounds and is characterized by the breaking and forming of chemical bonds synchronously. It is based on nucleophilic substitution.
The reaction between ethyl bromide and sodium cyanide is SN2 as it has a strong nucleophile and acetone act as a polar solvent. Ethyl bromide is an alkyl halide that has leaving group for the substrate.
Therefore, it is an SN2 reaction mechanism.
Learn more about the SN2 mechanism, here:
https://brainly.com/question/12242526
#SPJ4
list the processes that release carbon into the atmosphere
Answers
Answer:
Fossil fuels, such as coal, oil, or natural gas, release carbon back into the atmosphere.
The processes would be decomposition, diffusion, erosion, respiration, and combustion.
Explanation:
Hope this helped?
Here are two different reactions:
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) + 213 kcal
- N2(g) + O2(g) + 45 kcal → 2NO(g)
Which of the above reactions might have taken place in the beaker? Support your answer with evidence.
Answers
The reaction that does not occur in a beaker is;
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) + 213 kcal
Why does combustion reaction not occur in a beaker?In a beaker, it is unlikely that all of these conditions will be met for a combustion reaction to occur. For example, there may not be enough fuel present in the beaker to sustain a combustion reaction, or there may not be enough oxygen to support the reaction.
Additionally, an ignition source such as a spark or flame is usually necessary to initiate the reaction, which is unlikely to be present in a beaker.
Learn more about combustion:https://brainly.com/question/15117038
#SPJ1
Which model of the atom has electrons traveling in specific paths around the nucleus?
A. Thomson’s model
B. Rutherford’s model
C. Bohr’s model
D. Dalton’s model
Answers
Answer:
C
Explanation:
this is because the rest talk about the findings of protons,neutrons and electrons.
How many atoms are present in 2.79 mol of NH3?
Answers
Answer:
2.79 mol NH3 have 2.79 mol of N atoms. Answer is 1.68*10²⁴ N-atoms
Calculate the average atomic mass of element Q, if the isotopic composition of element Q is as follows: Q-107 has an abundance of 78%, and Q-110 has an abundance of 22%. Type your answer rounded to two decimal places.
Answers
Answer:
5
Explanation:
roughly how long is the entire life cycle of a star like our Sun? Explain how
you worked it out.
Answers
Answer:
Star is a cycles two
Sana maka tolong
the molecular weight of sulfadiazine is 250 g/mol. what volume of dmso is needed to make a 20 mm stock solution of sulfadiazine if you weight out 4.7 mg of this compound
Answers
The volume of DMSO needed to make a 20 mm stock solution of sulfadiazine, when weighing out 4.7 mg of this compound, is approximately (1.88 x 10^-5 mol) / 20 mm * (1 mL/1.1 g) = 0.085 mL.
To calculate the volume of DMSO needed to make a 20 mm stock solution of sulfadiazine, we can use the equation:
C1V1 = C2V2
Given:
Molecular weight of sulfadiazine = 250 g/mol
Weight of sulfadiazine = 4.7 mg
First, we need to convert the weight of sulfadiazine from milligrams to grams:
4.7 mg = 0.0047 g
Next, let's calculate the number of moles of sulfadiazine:
moles = mass / molecular weight
moles = 0.0047 g / 250 g/mol = 1.88 x 10^-5 mol
Now, let's plug the values into the equation:
C1V1 = C2V2
20 mm = (1.88 x 10^-5 mol) / V2
To solve for V2 (volume of DMSO), we rearrange the equation:
V2 = (1.88 x 10^-5 mol) / 20 mm
Now, let's convert the volume to milliliters by using the density of DMSO:
1 mL of DMSO = 1.1 g
V2 = (1.88 x 10^-5 mol) / 20 mm * (1 mL/1.1 g)
Therefore, the volume of DMSO needed to make a 20 mm stock solution of sulfadiazine, when weighing out 4.7 mg of this compound, is approximately (1.88 x 10^-5 mol) / 20 mm * (1 mL/1.1 g) = 0.085 mL.
To know more about sulfadiazine visit:-
https://brainly.com/question/30591109
#SPJ11
How much gram of base will be in solution of 2.3l of koh having normality 0.6n.
Answers
There are 77.27 grams of KOH present in the solution.
To determine the amount of grams of base present in a solution of 2.3L of KOH with normality 0.6N, we first need to understand the relationship between normality, volume, and the molecular weight of the compound.
Normality (N) is defined as the number of equivalents of solute per liter of solution. In this case, we know that the normality of KOH is 0.6N.
The molecular weight of KOH is 56.11 g/mol. This means that one mole of KOH weighs 56.11 grams.
To calculate the amount of base present in the solution, we can use the formula:
grams of base = normality x volume x molecular weight
Substituting the values given in the question, we get:
grams of base = 0.6N x 2.3L x 56.11 g/mol
Simplifying the expression, we get:
grams of base = 77.27 g
Therefore, there are 77.27 grams of KOH present in the solution.
To know more about solution visit :-
https://brainly.com/question/25326161
#SPJ11
( Brainliest + thanks! )
The ocean is made mostly of:
A) Carbon and oxygen
B) Hydrogen and carbon
C) Calcium and nitrogen
D) Hydrogen and oxygen
Answers
Answer: The ocean is made mostly of: D) Hydrogen and oxygen.
Explanation: The most abundant elements in the ocean by proportion of mass in percent are oxygen (85.84%), hydrogen (10.82%), chlorine (1.94%), sodium (1.08%), magnesium (0.13%), sulfur (0.09%), calcium (0.04%), potassium (0.04%), bromine (0.007%), carbon (0.003%), and boron (0.0004%).
Hope this helped!
9th grade...Chemistry help needed

Answers
Answer:
1). Scientific
2). Plating the diluted bacteria on media that supports the growth of only living micro organisms. Statistically accurate.
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
In this nuclear reaction equation, oxygen decays to form nitrogen. which equation correctly describes this decay? superscript 15 subscript 8 baseline upper o right arrow superscript 15 subscript 7 baseline upper n superscript 0 subscript 1 baseline e superscript 15 subscript 8 baseline upper o superscript 0 subscript 1 baseline e right arrow superscript 15 subscript 7 baseline upper n superscript 15 subscript 8 baseline upper o right arrow superscript 15 subscript 7 baseline upper n superscript 0 subscript negative 1 baseline e superscript 15 subscript 7 baseline upper o right arrow superscript 15 subscript 7 baseline upper n superscript 0 subscript 1 baseline e
Answers
In this nuclear reaction equation, oxygen decays to form Nitrogen. The equation which correctly describes this decay is 15/8 O → 15/7 N + 0/1 e.
What is an atomic reaction?Nuclear reaction or Nuclear reaction, in Nuclear Physics, is any reaction in which one or more atomic nuclei are modified, where two or more atoms unite or an atom undergoes nuclear fission. Such a reaction should not be confused with a chemical reaction, it occurs with the peripheral electrons of the atom.
Thus, the equation which correctly describes this decay is 15/8 O → 15/7 N + 0/1 e.
See more about nuclear reaction at brainly.com/question/12649087
#SPJ4
Answer:
15/8O --> 15/7N + 0/1e
Explanation:
edg
What kind of organic compound is ethanoic acid?
Answers
Answer:
Acetic acid , systematically named ethanoic acid , is a colourless liquid organic compound with the chemical formula CH3COOH.
Explanation:
What is one energy transformation that is taking place in the photo?
radiant energy to thermal energy
thermal energy to nuclear energy
chemical energy to thermal energy
radiant energy to chemical energy

Answers
Answer:
Radiant energy to chemical energy
Explanation:
Got it right on edge 2021
One energy transformation that is taking place in the photosynthesis process is radiant energy to chemical energy.
What is energy transformation?Energy transformation is the process in which one form of energy converted into another form of energy.
What is photosynthesis process?Photosynthesis process is defined as the process in which plants makes their own food by using the carbon dioxide, water and sunlight in the chlorophyll.
The balanced chemical equation of photosynthesis process is
6CO2 + 6H2O → C6H12O6 + 6O2.
Since, it have 6 atoms of carbon, 12 atoms of hydrogen and 18 atoms of oxygen on both side. So, it is balanced.
Since, sunlight which it take is in the form of radiant and convert it into chemical energy.
Thus, we concluded that the energy transformation that is taking place in the photosynthesis process is radiant energy to chemical energy.
learn more about photosynthesis process:
https://brainly.com/question/26568636
#SPJ2
Pls help!
Is volume equal to mass over density?
Answers
density = mass/volume
volume= mass/density
Yes, you're correct.
Answer:
Volume isn't equal to mass.
Explanation: Mass is weight, volume is like how much something can hold.
Some materials are phosphorescent, meaning that they can absorb light energy from the Sun, and then re-emit this energy later. Many objects people use today are phosphorescent, including glow-in-the-dark plastics. Some plastic objects have phosphorescent materials in them, which causes them to glow after they are exposed to light. In 1896, one year after x-rays were discovered, scientist Henri Becquerel hypothesized that uranium might work in this way as well. So he exposed a sample of uranium to sunlight, and then was pleased to discover that the uranium continued to emit energy even after it was taken out of the light. However, after several straight days of cloudy skies over his laboratory, Becquerel was shocked to discover that the energy kept coming from the uranium, even without sunlight. What had Becquerel accidentally discovered?
Answers
Answer:
Explanation:
After the Becquerel came to know the discovery of X-ray by Roentgen Becquerel began looking for a connection between the phosphorescence and the newly discovered X-rays. His thought was that phosphorescent uranium salts might absorb sunlight and reemit it as x rays.
To experiment this he exposed a sample of uranium to sunlight, and then was pleased to discover that the uranium continued to emit energy even after it was taken out of the light. However, after several straight days of cloudy skies over his laboratory, Becquerel was shocked to discover that the energy kept coming from the uranium,
He reported that the uranium salts emitted radiation without any stimulation from sunlight. He did further tests to confirm that sunlight was indeed unnecessary, that the uranium salts emitted the radiation on their own.
Initially he thought the effect was due to particularly long-lasting phosphorescence, but he soon discovered that non-phosphorescent uranium compounds exhibited the same effect. After that he announced that the element uranium was indeed what was emitting the radiation.
At first he also believed his rays were similar to x-rays, but his further experiments showed that unlike x-rays, which are neutral, his rays could be deflected by electric or magnetic fields which was against his expectations. Finally “Radioactivity” term was given to describe this new phenomenon.
This story of becquerel is well known example of accidental discovery.
what is the relationship between the calculated/theoretical number of ions/particles in solution and the actual number of particles in solution?
Answers
The relationship between the calculated/theoretical number of ions/particles in solution and the actual number of particles in solution depends on several factors, such as the accuracy and precision of the analytical method used to determine the number of particles.
In reality, the actual number of ions or particles in a solution can vary from the calculated number due to a number of factors. For example, ions can interact with each other and form complexes, or they can be adsorbed onto the surface of solids or other surfaces in the solution. Additionally, ions can be lost from solution due to precipitation, and they can be present in the solution in different forms (such as free ions, ion pairs, or hydrated ions).
Therefore, while the calculated number of ions or particles in solution provides a useful starting point for understanding the behavior of a solution, it is important to keep in mind that the actual number of ions or particles in solution can differ from the calculated number, and that other factors can influence the behavior of the solution.
Learn more about solution here:
https://brainly.com/question/30620786
#SPJ4
how does one determine a molecular formula from the epirical formula
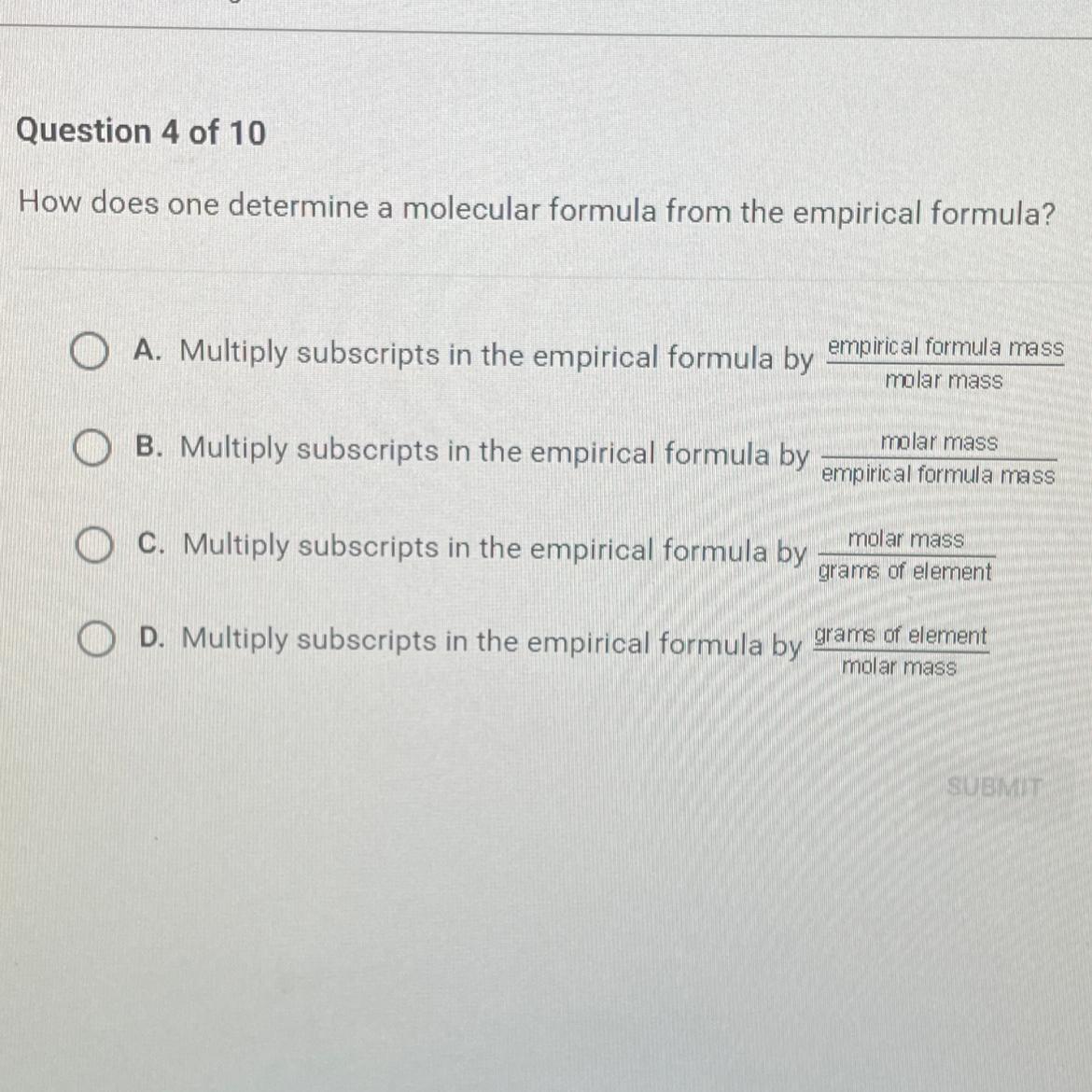
Answers
Answer:
C
Explanation:
In a converter, 9.33 kg of SO3 is fed and allowed to come into contact with a certain amount of 91.34% H2SO4 to produce 4.71% oleum. How much oleum was produced in kg? Use the following molecular weights: 80 kg/kmol SO3, 98 kg/kmol H2SO4.
Answers
To solve this problem, we need to determine the amount of oleum produced when 9.33 kg of SO3 reacts with a certain amount of 91.34% H2SO4 to produce 4.71% oleum.
Let's first calculate the mass of H2SO4 present in the initial solution. Since the solution is 91.34% H2SO4, we have:
Mass of H2SO4 = 91.34% * 9.33 kg = 8.51 kg
Next, we can calculate the mass of oleum produced. Since the oleum concentration is 4.71%, we have:
Mass of Oleum = 4.71% * 9.33 kg = 0.439 kg
Therefore, approximately 0.439 kg of oleum was produced.
to learn more about specific volume, visit:
brainly.com/question/10831664
#SPJ11
Gandhiji came back to india _____
a) 1915
b) 1885
c) 1526
d) 1877
Answers
I hope this helps :)
Answer:
January 9th 1915
Explanation:
4th attempt Which of the following phase transitions are exothermic? Choose all that apply. Choose one or more: A. sublimation ? B. freezing C. melting ? D. d«position E. condensation F. evaporation
Answers
Sublimation and melting are exothermic phase transitions. Exothermic describes a process or reaction that releases energy in the form of heat.
Here correct answers is A and C
During sublimation, a solid goes directly to a gas state without passing through the liquid state. During melting, a solid turns into a liquid as energy is released in the form of heat.
Conversely, endothermic processes absorb energy in the form of heat. Examples of endothermic phase transitions include freezing, deposition, condensation, and evaporation. During freezing, a liquid turns into a solid as energy is absorbed in the form of heat.
During deposition, a gas turns into a solid as energy is absorbed in the form of heat. During condensation, a gas turns into a liquid as energy is absorbed in the form of heat. During evaporation, a liquid turns into a gas as energy is absorbed in the form of heat.
Know more about endothermic processes here
https://brainly.com/question/29555731#
#SPJ11
i will give brainliest!! plz no links i wiill report
what experimentation,events and/or discoveries led to the development of Hydrogels?
Answers
During the winter in grassland ecosystems, rabbits and ground hogs both hibernate in holes. Due to an extremely wet summer much of the ground cannot be used for winter hibernation burrows. This causes an increase in competition between rabbits and ground hogs. Explain a structural and a behavior adaptation that the rabbits could adapt to deal with these new pressures.
Answers
Explanation:
They have to battle them dang hogs and get them burrows before them.
6. What is the optimum pH range for blood? What happens if the blood pH is outside this range? (C 3 marks) 7. What are the 3 mechanism that control body pH? (K/U 3 marks) 8. How does blood control pH?
Answers
The optimum pH range for blood is approximately 7.35 to 7.45. Deviations from this range can have serious consequences on physiological processes. The body employs three mechanisms to control pH: buffers, respiratory regulation, and renal regulation. Blood plays a crucial role in maintaining pH homeostasis by utilizing these mechanisms.
The optimum pH range for blood is tightly regulated between 7.35 and 7.45. If the blood pH deviates from this range, it can disrupt normal physiological processes. For example, if the blood becomes too acidic (pH below 7.35), a condition called acidosis occurs.
Acidosis can lead to impaired enzyme function, decreased oxygen delivery to tissues, and disruption of the central nervous system. On the other hand, if the blood becomes too alkaline (pH above 7.45), a condition called alkalosis occurs. Alkalosis can result in muscle twitching, confusion, and even seizures.
To maintain the pH within the optimal range, the body employs three primary mechanisms: buffers, respiratory regulation, and renal regulation. Buffers are chemical substances that can accept or donate hydrogen ions to resist changes in pH. They can bind excess hydrogen ions when blood becomes acidic or release hydrogen ions when blood becomes alkaline.
The respiratory system regulates pH by adjusting the levels of carbon dioxide (\(CO_{2}\)) in the blood through changes in breathing rate and depth. By altering the amount of \(CO_{2}\), the body can regulate the concentration of carbonic acid (\(H_{2}\)\(CO_{3}\)) and thus control pH. The kidneys play a crucial role in long-term pH regulation by selectively reabsorbing or excreting bicarbonate ions (\(HCO_{3}\)-) and hydrogen ions (H+) in the urine.
Hence, the optimum pH range for blood is approximately 7.35 to 7.45. Deviations from this range can lead to acidosis or alkalosis, disrupting physiological processes. The body controls pH through buffers, respiratory regulation, and renal regulation. Buffers resist pH changes, the respiratory system regulates \(CO_{2}\) levels to control carbonic acid concentration, and the kidneys selectively reabsorb or excrete bicarbonate and hydrogen ions to maintain pH homeostasis.
Learn more about pH
https://brainly.com/question/12609985
#SPJ11
I need help with this pleaseYou are cooking a dinner and the recipe calls for chicken broth. You realize that you don’t have a can of liquid broth, but you have the dried cube form of chicken broth that can be dissolved in water.

Answers
Answer
crush the cubes of broth, add warm water and stir the container.
Explanation
The FASTEST way to make the chicken broth with the cubes you have will be to increase the surface area of the cubes broth by crushing and raise the temperature of the cubes broth by adding warm water and by stirring the container.
Hence, the correct answer to your question is:
crush the cubes of broth, add warm water and stir the container.