Billy decides to test the factors that affect plant growth. he uses sunflowers, daisies, and bean plants. he gives some of the plants fertilizer and other plants only water. he plants the sunflowers in a garden outside but plants the daisies and bean plants in small containers inside his house. he measures the height of the plants each day using a ruler. what is wrong with billy's experiment?
Answers
Billy conducted an experiment to check the growth of different types of plants in different factors. He uses plants like sunflowers, daisies, and bean plants.
He planted the sunflower outside in a garden and plant the daisies and bean plants inside his house in a small container. He provides some of the plants only water and other plants only fertilizers. Then he started measuring the height of the plants everyday using a ruler.
The mistake with the Billy's experiment is that he uses too many manipulated variables. In his experiment he uses too many plants and flowers. Also, he uses fertilizers in only some of the plants and not in others.
Know more about Experiment on Plants: -https://brainly.com/question/16764271
Related Questions
Ifa container of nitrogen and oxygen gas holds 2. 50 atm of N2 gas and 1. 50 atm of O2 gas, what
is the total pressure inside the container?
Answers
The total pressure inside the container is 4.00 atm. This is because the total pressure of a gas mixture is equal to the sum of the individual pressures of each gas present. In this case, we have 2.50 atm of N2 gas and 1.50 atm of O2 gas.
When these two values are added together, we get the total pressure of 4.00 atm. This total pressure is also known as the partial pressure of the gas mixture.
The partial pressure of the gas mixture is the sum of the individual partial pressures of each gas present. Since the total pressure of a gas mixture is equal to the sum of the individual pressures of each gas present, the total pressure in the container is 4.00 atm.
Know more about Total pressure here
https://brainly.com/question/30255561#
#SPJ11
most chemical reactions in organisms are regulated by organic catalysts known as
Answers
Answer:
An enzyme is a substance that acts as a catalyst in living organisms, regulating the rate at which chemical reactions proceed without itself being altered in the process. The biological processes that occur within all living organisms are chemical reactions, and most are regulated by enzymes.
Explanation:
Hope this helps...
Mendeleev placed thallium (Tl) in the same group as lithium (Li), sodium (Na), potassium (K), rubidium (Rb), and cesium (Cs). However, the modern periodic table doesn’t place thallium in this group. Use the periodic table to find the properties of thallium. Explain why Mendeleev might have grouped thallium in the same group as lithium and sodium. Also explain how thallium is different from the other five elements.
Answers
Answer:
When observing how thallium reacts with the air of the earth's atmosphere, its hardness or resistance resembled sodium, it was not investigated further to classify it correctly
Explanation:
Now it is known that they contain different numbers of valence electrons and that thallium is a heavy metal like lead and that they have similar characteristics except for their melting point where thallium is higher.
Mendeleev might have grouped thallium in the same group as lithium and sodium due to many reasons:
Since, all are metals so they need to be placed in the same group.The particular group is referred to (group one) is actually the most reactive metals on the periodic table. These are the elements that most easily loose their electron from their outer valence shell. Thus each element can easily give up their valence electron in a reaction to form a positive ion. These are the most easily reactive in this way.When observing how thallium reacts with the air of the earth's atmosphere, its hardness or resistance resembled sodium, it was not investigated further to classify it correctly.Now it is known that they contain different numbers of valence electrons and that thallium is a heavy metal like lead and that they have similar characteristics except for their melting point where thallium is higher.
Learn more:
brainly.com/question/17169639
Consider the following elements: Ga, Sr, P, F, Ge, Rb. Arrange them in order of the following.
A. increasing atomic radius
B. decreasing first ionization energy
Answers
The arrangement of the given elements in increasing order of atomic radius is F< P< Ge< Ga< Sr< Rb and in terms of decreasing first ionization energy is F> P> Ge> Ga> Sr> Rb.
A. Atomic radius refers to the distance from the center of an atom's nucleus to its outermost electrons.The atomic radius generally increases down the group as energy levels are added making each successive atom in a group larger. In moving across a period the atomic radius decreases because the nuclear charge increases as protons are added.
Thus, F has the smallest atomic radius among these elements whereas Rb has the largest.
B. Ionization energy is defined as the amount of energy needed to remove one electron from an atom in its gaseous state. It is a measure of how tightly or loosely an outermost electron is bound. Elements that readily give up electrons will have the lowest first ionization energy while those that hold their outermost electrons most strongly will have the highest first ionization energy. Down the group, ionisation energy decreases due to increase in the size of atom whereas along the period, ionization energy increases as it becomes difficult to remove an electron form the outermost shell due to decrease in the size of the atom.
Thus, here F has the highest first ionization energy whereas Rb has the lowest.
Therefore, increasing order of atomic radius is F< P< Ge< Ga< Sr< Rb and decreasing order of first ionization energy is F> P> Ge> Ga> Sr> Rb.
To learn more about ionization energy :
https://brainly.com/question/30831422
#SPJ11
u want 100 points well do it then
Answers
Answer: a
Explanation: a hypoth is a if then statement meaning its not certain and a is the one mostly in that structure
Ordered sodium amytal 0.1 gm IM stat Available sodium amytal 200mg/3ml How many mls would you give IM?
Answers
To get a dose of 0.1 gm (100 mg), 1.5 ml of sodium amytal solution must be injected intramuscularly (IM).
What is sodium amytal ?We can use the available concentration and the desired dose.
Given
Available sodium amytal concentration: 200 mg/3 mlDesired dose: 0.1 g (which is equivalent to 100 mg)First, we need to convert the desired dose from grams to milligrams:
0.1 g = 100 mg
Now, we can set up a proportion to find the volume of solution needed:
(100 mg) / (200 mg) = (x ml) / (3 ml)
Cross-multiplying and solving for x:
100 mg * 3 ml = 200 mg * x ml
300 mlmg = 200 mlmg
x ml = (300 ml*mg) / (200 ml)
x ml = 1.5 ml
So, To get a dose of 0.1 gm (100 mg), 1.5 ml of sodium amytal solution must be injected intramuscularly (IM).
Learn more about proportion here :brainly.com/question/870035
#SPJ1
IUPAC NAME of the following

Answers
The IUPAC name of compound is propanoic acid.
What is IUPAC name?The purpose of the IUPAC system of nomenclature is to establish an international standard of naming compounds to facilitate communication.
The goal of the system is to give each structure a unique and unambiguous name, and to correlate each name with a unique and unambiguous.
Alkanes are the family of saturated hydrocarbons, that is, molecules containing carbon and hydrogen connected by single bonds only. These molecules can be in continuous chains (called linear or acyclic), or in rings (called cyclic or alicyclic). The names of alkanes and cycloalkanes are the root names of organic compounds.
Therefore, The IUPAC name of compound is propanoic acid.
To learn more about IUPAC, refer to the link:
https://brainly.com/question/8644699
#SPJ1
Worth many points (timed test)
A 54.2 g sample of an unknown metal is heated to 48.00 degrees Celsius.
It is then placed in a coffee-cup calorimeter filled with water.
The calorimeter and the water have a combined mass of 183.1 g and an overall specific heat of 1.34 cal/g•°C.
The initial temperature is 10.2°C when the metal is added.
The system reaches a final temperature of 23.00 °C.
(show work)
Answers
Answer:
2.335 J/g*degrees celcius
Explanation:
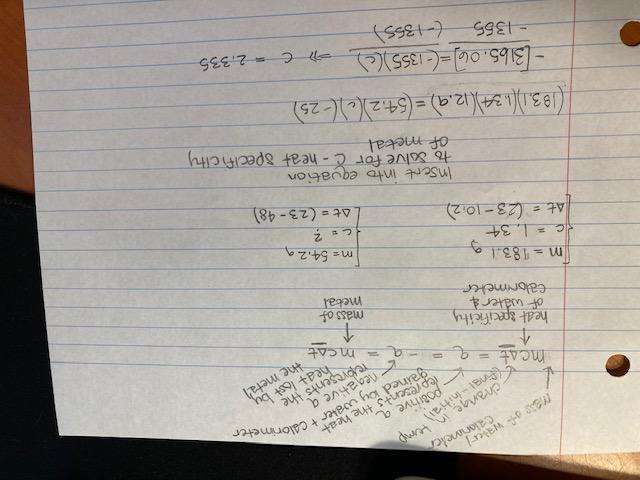
does the volume of a liquid change
Answers
Answer:
Yes it does change
Explanation:
consider a reaction that produces a significant amount of hydrogen ion and is to be carried out a ph 7. only two acids are available for making the buffer solution. the pka values for acids a and b are 6.3 and 7.3, respectively. which acid would serve as the optimum buffer for this reaction? or would carrying out the reaction in water simply serve as well?
Answers
Consider a reaction that produces a significant amount of hydrogen ion and is to be carried out a pH 7. The acid would serve as the optimum buffer for this reaction is the acid A.
The pH = 7
The pka value of the acid A = 6.3
The pka value of the acid B = 7.3
The buffer range is given as :
For acid A = (pka + 1 ) to (pka - 1)
The optimal buffer range is the range of the buffer in the dissociation constant of the weak acid to the buffer pka plus or the minus pH unit.
To learn more about pH here
https://brainly.com/question/21801382
#SPJ4
Write a balanced chemical equation for the dissociation of lithium bromide in water.
Answers
The balanced chemical equation is :
LiBr (aq) + H2O (l) → LiOH (aq) + HBr (aq) .
Explain the balanced chemical equation?To balance a chemical equation, add coefficients to the symbols or formulas as needed so that the same number of each type of atom occurs in both reactants and products.There is a strategy that can help you balance equations faster. It is known as balancing by inspection. Essentially, you take the number of atoms on each side of the equation and add coefficients to the molecules to balance out the number of atoms.Lithium bromide, abbreviated LiBr, is a chemical compound. It is water, alcohol, and ether soluble. It is made by reacting hydrobromic acid with lithium hydroxide.To learn more about balancing chemical equation refer to :
https://brainly.com/question/26694427
#SPJ4
11. How is the atomic emission spectrum of an element produced?
Answers
Answer:
Atomic emission spectra are produced when excited electrons return to the ground state. When electrons return to a lower energy level, they emit energy in the form of light.
Explanation:
Model the chemical reaction between methane and oxygen. Drag the atoms from the reactants to form the products in the reaction. Use the equation to help you.
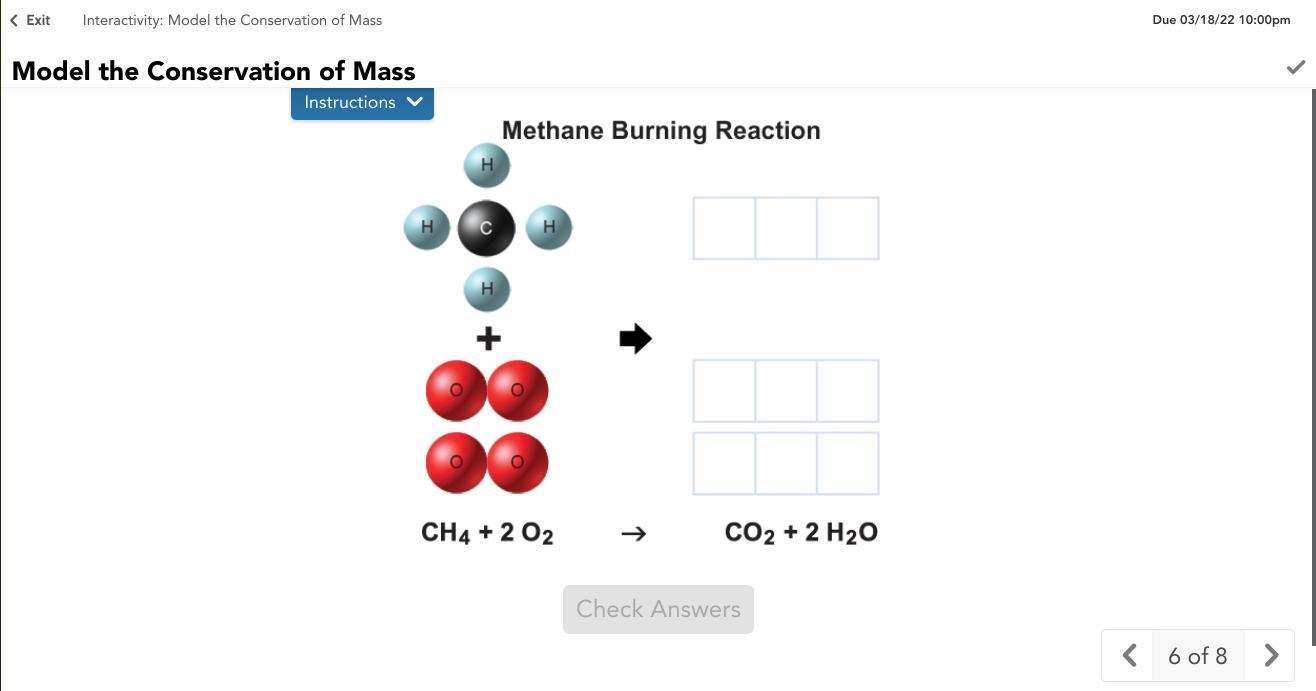
Answers
Answer:
Answer is in the image attached :)

How can you determine the charge of an Ion knowing the number of protons and neutrons?
Answers
For example, an ion has 8 number of protons and 5 number of neutrons. There is more number of protons (+) than neutrons (-), therefore the ion will have a positive charge. Vice versa, if there is more number of neutrons than number of protons, means the charge will be negative.
When the humidity is high, the change of precipitation is (high or low).
Answers
Answer:
low, when humidity is low, the chance of precipitation is high.
(so the answer is low :)
Explanation:
What is an ensemble experiment? how might single-molecule experimental results vary from ensemble experimental results?
Answers
An ensemble experiment is an experiment to assess the average size of many molecules, which depends on the size of each molecule.
What is an ensemble experiment?An ensemble experiment is an experimental procedure where the size of molecules is expressed as an assemble value.
This type of experiment (ensemble experiment) may vary according to the size of a specific molecule that may alter the outcome (i.e., the average size).
In conclusion, an ensemble experiment is an experiment to assess the average size of many molecules, which depends on the size of each molecule.
Learn more about experiments in chemistry here:
https://brainly.com/question/26150306
#SPJ1
What is the mass of hydrogen in 5.5 mol of Ca(OH)2?
Answers
Answer:
12*2*6.022*10^23. Atoms of hydrogen.
Explanation:
First of all if u know that 1 molecule of calcium hydroxide contain 2 hydrogen atom and 1 mole of calcium hydroxide contain 6.022*10^23 molecules of calcium hydroxide.
Hence 1 mole of calcium hydroxide contain 2*6.022*10^23 atom of hydrogen.
So 12 mole of calcium hydroxide contain =12*2*6.022*10^23. Atoms of hydrogen.
A chemist is trying to classify an unknown substance as either a metal or nonmetal. What question should the chemist use to help classify the material?
A. Does the material feel hard to the
touch?
B. Does the material feel rough or smooth?
C. Is the material a good conductor or a poor conductor?
D. Will the material float in water?
Please Help me!!!
Answers
Answer:
c
Explanation:
metals are good conductors, while non metals are not good conductors
why does salt water have more cohesion than tap water
Answers
Answer:
Cohesion exists because of the polarity of water. The water has a dipole that causes it to act like a magnet, attracting other water molecules to it. ... The salt water has a much lower cohesion than plain water so it's attractive forces are less than plain water.
the two isotopes of uranium, 238u and 235u can be separated by diffusion of the corresponding uf6 gases. calculate the ratio of the rates of diffusion of 238uf6 to 235uf6 at room temperature. the molar mass of fluorine can be found on the periodic table. molar mass 235u: 235.0439 g/mol molar mass 238u: 238.0508 g/mol which gas diffuses more quickly? 15. (10 points) liquid ammonium (boiling point
Answers
This means that 238UF6 diffuses slightly slower than 235UF6, with a ratio of 0.976.
The ratio of the rates of diffusion of 238UF6 to 235UF6 can be calculated using Graham's law of diffusion, which states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass.
Rate of diffusion of 238UF6/Rate of diffusion of 235UF6 = Square root of (molar mass of 235UF6/molar mass of 238UF6)
Rate of diffusion of 238UF6/Rate of diffusion of 235UF6 = Square root of (235.0439/238.0508)
Rate of diffusion of 238UF6/Rate of diffusion of 235UF6 = 0.976
This means that 238UF6 diffuses slightly slower than 235UF6, with a ratio of 0.976. This difference in diffusion rates can be used to separate the isotopes using a diffusion-based process.
To know more about Graham's law of diffusion, refer here:
https://brainly.com/question/24553716#
#SPJ11
aluminum hydroxide molecular weight step by ste
Answers
Carbon dioxide molecules (select all that apply)
Group of answer choices
Protect the Earth from all of the harmful Ultraviolet (UV) radiation
Absorb most of the shortwave radiation emitted from the Sun
Are one of the most abundant constituents of Earth's atmosphere
Can move in many ways, thus absorbing and emitting infrared radiation
Answers
Carbon dioxide molecules can absorb and emit infrared radiation, and they are one of the most abundant constituents of Earth's atmosphere.
Thus, the correct options are:d) Are one of the most abundant constituents of Earth's atmospheree) Can move in many ways, thus absorbing and emitting infrared radiation
Carbon dioxide is a trace gas present in the Earth's atmosphere. It's a vital component of Earth's carbon cycle, which helps to regulate Earth's temperature and support life as we know it. Carbon dioxide molecules are one of the most common gases in the atmosphere, accounting for around 0.04% of the Earth's atmosphere.
The greenhouse effect is caused by carbon dioxide, methane, and other greenhouse gases. When the Sun's energy reaches the Earth's surface, it is absorbed and then radiated back into space as infrared radiation. Greenhouse gases absorb this radiation and trap it in the atmosphere, which causes the Earth's temperature to rise and the climate to change.
Carbon dioxide molecules are capable of absorbing and emitting infrared radiation due to their molecular structure, which consists of one carbon atom and two oxygen atoms. This property of carbon dioxide is the main reason it's classified as a greenhouse gas.
To know more about Carbon dioxide molecules visit:
https://brainly.com/question/12770212
#SPJ11
what is the molecular geometry of co2 ? enter the molecular geometry of the molecule. view available hint(s)for part b part c what is the molecular geometry of seh2 ? enter the molecular geometry of the molecule. view available hint(s)
Answers
The molecular geometry of CO₂ is linear, and the molecular geometry of SeH₂ is bent.
The molecular geometry of a molecule is the arrangement of its atoms in space, taking into account the number of atoms, electron pairs, and lone pairs on the central atom. The molecular geometry of a molecule is determined by its bonding, shape, and size.
Molecular geometry is also known as the shape of molecules. CO₂ is a linear molecule with a carbon atom in the center and two oxygen atoms on either side. Each oxygen atom has two non-bonding pairs of electrons, and the carbon atom has no non-bonding electrons. As a result, the molecular geometry of CO₂ is linear.
SeH₂ is a bent molecule with a central selenium atom and two hydrogen atoms. The lone pair of electrons on the selenium atom causes the molecule to be bent, giving it a shape similar to that of water. The molecular geometry of SeH₂ is bent, with a bond angle of approximately 98 degrees.
Learn more about molecular geometry here.https://brainly.com/question/29650255
#SPJ11
A lake has been infected by some type of new algae that is unknown. Every single day the amount of surface area that the algae takes up doubles. Day 1 has a certain amount, day 2 it is 2x that amount. It takes 87 days for the entire lake to be overrun by this new algae. How many days does it take to cover half of the lake? Show your work and explain your thinking. In science we need to be able to justify our answers.
hint: It is not the "obvious" answer.
Answers
Answer:
It takes 86 days take to cover half of the lake
Explanation:
In the day #1, the amount of the algae is X,
In the day #2 is 2X
In the day #3 is 2*2*X = X*2²
...
In the day #n the amount of the algae is X*2^(n-1)
Assuming X = 1m³. In the day 87, the area infected was:
1m³*2^(87-1)
7.74x10²⁵m³ is the total area of the lake
the half of this amount is 3.87x10²⁵m³
The time transcurred is:
3.87x10²⁵m³ = 1m³*2^(n-1)
Multiplying for 5 in each side:
ln (3.87x10²⁵) = ln (2^(n-1))
58.9175 = n-1 * 0.6931
85 = n-1
86 = n
It takes 86 days take to cover half of the lakeWhich method could you use to
encourage more product, SO2, to form
from the reaction below?
4558 kJ+2SO 3 (g)2S*O_{2}(g) + O{2}(g)
Cool the system
Decrease the volume of the container
Remove SO3
Remove O2
Answers
The method which increases the formation of SO₂ is remove O₂ from the system. Hence, option d is correct.
What is chemical equilibrium ?The state at which the rate of forward reaction and backward reaction is becomes equal is called equilibrium. If any disorder occurs in the system, the system itself balance that and shifts to a new equilibrium state.
By decreasing the concentration of reactants, the system shifts to the backward direction to restore the reactants. If reactant concentration is increased forward reaction takes place to consume the reactants and form products.
Here, SO₂ formed in the forward reaction. Removal of oxygen makes the system shifts to forward direction and encourage the production of SO₂.
Therefore, option d is correct.
Find more on chemical equilibrium:
https://brainly.com/question/4289021
#SPJ1
Answer:Remove O2
Explanation:Acellus confirmed
Help I need help with this two!

Answers
The way in which inlets are impacted by high tides is:
High tides fill inlets with water so they become larger.What is the impact on inlets by high tides?
High tides can have a significant impact on inlets, as they cause the water level to rise and fill the inlet with seawater.
This can cause the inlet to become larger, as the water carries sediment and debris into the inlet, increasing its size and depth. This can also result in changes to the shape and position of the inlet, as the force of the water can cause erosion and deposition along the shoreline.
Overall, high tides can have both positive and negative effects on inlets, depending on the specific conditions and environmental factors involved.
Learn more about high tides at: https://brainly.com/question/2604305
#SPJ1
Cellular respiration occur within ______ of a cell.
Answers
Answer:
The mitochondria
Explanation:
Its where cellular respiration occurs
One of the reasons plants are important to us is because they reduce the amount of carbon dioxide in the atmosphere and increase the amount of oxygen, which we need to breathe. Explain how this statement relates to the chemical equation for photosynthesis.
Answers
Answer:
Photosynthesis is a phenomenon in which green plants such as algae, fungus etc containing chlorophyll use sunlight as a source of energy to convert carbon dioxide and water present in the atmosphere to form glucose which is used as plant food and oxygen which is liberated into the atmosphere for breathing.
The chemical equation for photosynthesis is:
\(6CO_2+6H_2O\rightarrow C_6H_{12}O_6+6O_2\)
where carbon dioxide and water are reactants and glucose and oxygen are products formed.
SOMEONE PLEASE HELP
Classify each of the following as either a pure substance or a mixture.
(a) Pop is composed of water, sugar, and carbon dioxide.
(b) Carbon dioxide is composed of carbon and oxygen chemically
combined.
(c) Sand is composed of white grains and black grains.
(d) The graphite at the centre of a pencil is composed of carbon.
Answers
Answer:
(a) Mixture
(b) Pure substance
(c) Mixture
(d) Pure substance
what is the iupac name for the compound shown below h3c-ch2-ch-ch2
Answers
The IUPAC name for the compound H₃C-CH₂-CH-CH₂ is 3-methylbutane.
Let's break down the naming process step by step :
The compound has four carbon atoms, so the root name will be "butane." The longest continuous carbon chain in the molecule contains four carbon atoms.Next, we need to identify any substituents or side groups attached to the main chain. In this case, there is a methyl group (-CH3) attached to the third carbon atom. Since it is attached to the third carbon, we use the prefix "3-methyl."Putting it together, we have "3-methylbutane," which accurately describes the structure of the compound. The prefix "3-" indicates the position of the methyl group, and "butane" represents the four-carbon main chain.To know more about the IUPAC name refer here :
https://brainly.com/question/16631447#
#SPJ11