Because of metallic bonding, when light is pointed at metals, they become
Oshiny
O malleable.
O ductile.
O conductive,
Answers
Owing to the electronic configuration of metals ,metallic bonding exists in metals because of which when light is pointed at them they become shiny.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/14283892
#SPJ6
Related Questions
non- acetone nail polish remover (ethyl acetate) molecular forces evaporation.T/F
Answers
Non-acetone nail polish remover, which typically contains ethyl acetate, does undergo evaporation. Ethyl acetate is a volatile organic compound with a relatively low boiling point.
It is known for its ability to evaporate quickly, making it an effective solvent in nail polish removers. The evaporation process occurs due to the intermolecular forces present in the ethyl acetate molecules. In ethyl acetate, there are two main intermolecular forces at play: dipole-dipole interactions and London dispersion forces. The presence of an oxygen atom and a carbonyl group in the molecule leads to a partial positive charge on the carbon and partial negative charge on the oxygen. This polarity allows for dipole-dipole interactions between neighboring ethyl acetate molecules.
Additionally, ethyl acetate molecules experience London dispersion forces, which arise from temporary fluctuations in electron distribution that induce temporary dipoles. These temporary dipoles can induce similar dipoles in neighboring molecules, leading to attractive forces. As the temperature increases, the kinetic energy of the molecules also increases. This results in an increased likelihood of molecules escaping from the liquid phase and transitioning into the gas phase through evaporation.
Therefore, due to the intermolecular forces present in ethyl acetate and its relatively low boiling point, non-acetone nail polish remover containing ethyl acetate does undergo evaporation.
Learn more about evaporation here:https://brainly.com/question/320765
#SPJ11
6. One way of obtaining pure water from muddy water is by A. filtration. B. dissolving the mud C.purification D. chlorination.
Answers
Answer:
A, Filtration
Explanation:
Once I did an Experiment when I took a funnel, filter paper and a beaker and muddy water.
I placed the funnel on the beaker and inserted the filter paper before the hole in the funnel.
I put the mud water and clean water came out, this is because the dirt got stuck to the filter paper due to the extremely small pores of the filter paper
Why is the death of a Star important for our life on Earth?
Answers
Explanation:
The reason why stars are so important is that they have helped humans navigate through Earth. When it was dark these stars would light up the sky giving people lightly. In addition, stars are very important because they make life on Earth. ... Earth would just be a rock with ice.
2KClO3 Right arrow. 2KCl + 3O2
How many moles of oxygen are produced when 2 mol of potassium chlorate (KClO3) decompose?
Answers
There are 3 moles of oxygen are produced when 2 mol of potassium chlorate (KClO3) decompose.
This indicates that 3 moles of oxygen gas are produced for every 2 moles of potassium chlorate that are decomposed. The reaction produces 3 moles of oxygen gas for every 2 moles of potassium chlorate that decomposes. Decomposing 2 moles of potassium chloride KClO3 produces 2 moles of potassium chloride KCl and 3 moles of oxygen O2.
Calculate the number of moles of hydrogen chloride produced from 11 moles of hydrogen. The yield of the reaction is 85%, which means that only 80% of the moles of product released are obtained. To get 3.50 moles of O2, or 85% of No. A mole to protect. Therefore 2.75 mol of KCLO3 is required. The only remaining units are moles of oxygen.
Learn more about Moles here:-https://brainly.com/question/15356425
#SPJ1
what is rtp in chemistry?
Answers
RTP stands for Room Temperature and Pressure.
One mole of any gas has a volume of 24 dm 3 or 24,000 cm 3 at rtp (room temperature and pressure). This volume is called the molar volume of a gas. This equation shows how the volume of gas in dm 3 at rtp is related to the number of moles: volume of gas at rtp = number of moles × 24.
Hope this helped!
On a cold, winter day, Sheena rubs her hands together to warm them up. How could Sheena actions be used to explain the Law of Conservation of Energy?
Answers
Answer:
When she rubs her hands together, it causes heat or friction
1. How many centimeters are in 13.4 inches?
Answers
34.036 Thats how many
Answer:
34.036
Explanation:
There are 2.54 centimeters in one inch so you multiply 13.4 and 2.54 to get 34.036.
which surface has most friction

Answers
An atom has a diameter of 2.00 Å and the nucleus of that atom has a diameter of 9.50×10−5 Å . Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere.
Answers
fraction of the volume of the atom that is taken up by the nucleus is 1.07x10⁻¹³Å³
Sphere volume is calculated by formula =
V=4/3(πr³) , V is volume , r is radius
Radius = diameter/2
Radius of atom = 2/2=1
Radius of nucleus = (9.50x10⁻⁵)/2 = 4.75x10⁻⁵
now we may calculate the volume for the atom and the nucleus
atom volume = (4/3) × 3.14 × (1 )³ = 4.186 ų
nucleus volume = (4/3) × 3.14 × (4.75× 10⁻⁵)³ = 4.48 × 10⁻¹³Å³
now the fraction of volume of the atom that is taken up by the nucleus:
fraction = nucleus volume / atom volume
fraction =4.48 × 10⁻¹³/4.186 = 1.07x10⁻¹³Å³
learn more about volume of atom and nucleus here_
https://brainly.com/question/13241621
#SPJ9
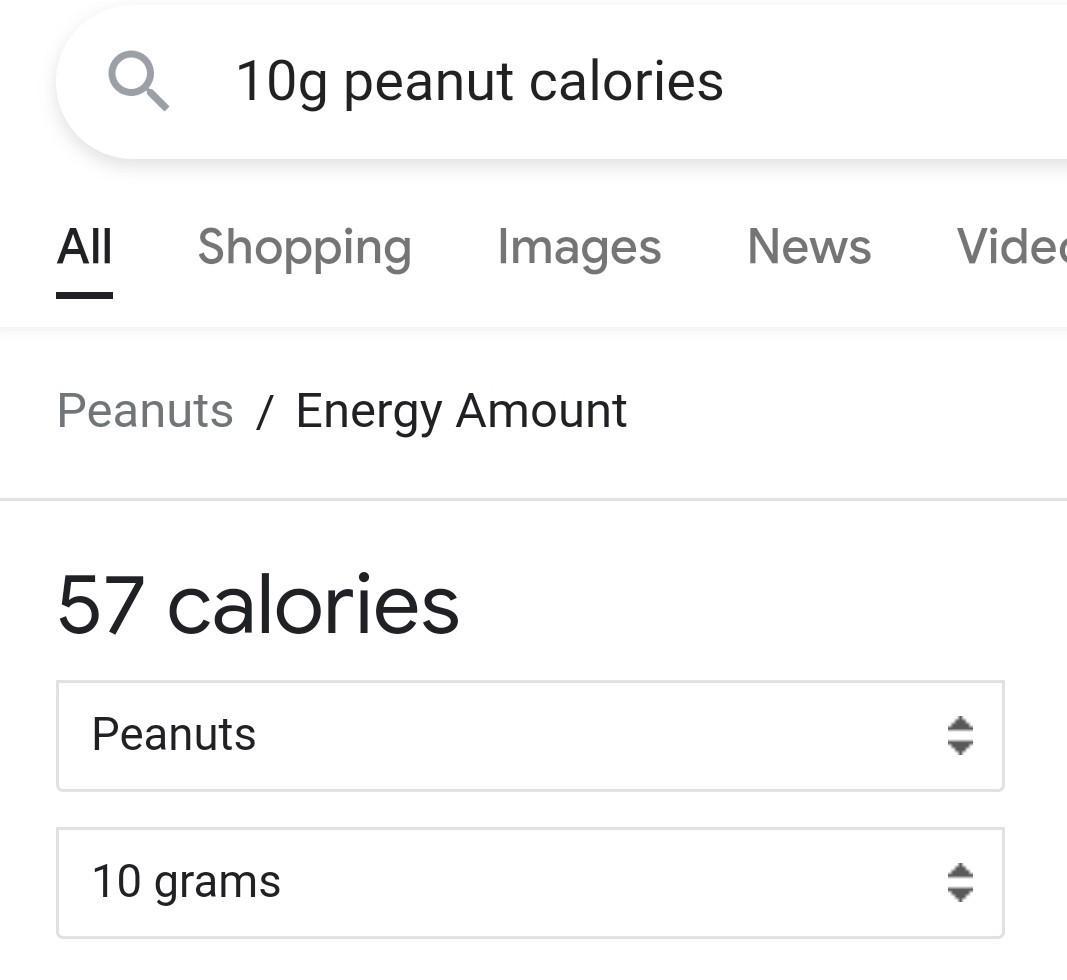
how many calories is in one peanut if the volume of water is 10 mL and the water temperature is rise 4 degrees celsius
Answers
Answer:
40.02 calories
Explanation:
V = 10 mL = 10g
we know t went up by 4°C, this is our ∆t as it is a change.
Formula that ties it together: Q = mc∆t
where,
Q = energy absorbed by water
m = mass of water
c = specific heat of water (constant)
∆t = temperature change
Q = (10 g) x (4.186 J/g•°C) x (4°C)
Q = 167.44 J
Joules to Calories:
167.44 J x 1 cal/4.184 J = 40.02 calories
(makes sense as in image it is close to the value).
Which of the folling ion or atom has smallest size?
a. CI
b. CI-
c. K
d. K+
Answers
why must the reaction mixture be cooled before water is added to precipitate the product?
Answers
Cooling the reaction mixture before adding water is done to prevent the mixture from boiling over and splattering.
During the reaction, the mixture may have been heated and generated a lot of heat. If water is added directly to the hot reaction mixture, it can cause rapid boiling and potentially lead to a dangerous situation.
Additionally, the product may be sensitive to high temperatures or heat shock, and rapid cooling can help prevent product degradation or decomposition. Therefore, it is important to cool the reaction mixture to a safe temperature before adding water to precipitate the product.
For more question on splattering click on
https://brainly.com/question/10889034
#SPJ11
How do I identify the reducing agent in a redox reaction
Answers
Answer:
If you can identify the species that was being oxidized, its species that contains the element on the other side is the reducing agent.
For example, in the equation Zn + Cu2+ --> Zn2+ + Cu, the zinc was oxidized, making Zn the reducing agent.
use the titration data to calculate the equilibrium concentration of the b 4 o 5 (oh) 42- and the na ions in each borax sample solution your group titrated
Answers
The equilibrium concentration of B4O5(OH)4^2- in each borax sample solution is X mol/L, and the equilibrium concentration of Na+ ions is Y mol/L.
To calculate the equilibrium concentrations of B4O5(OH)4^2- and Na+ ions, we need to use the titration data, which includes the initial volume and concentration of the NaOH solution, as well as the volume of NaOH required to reach the endpoint.
First, we can calculate the moles of NaOH used in the titration by multiplying the NaOH concentration by its volume used. Then, we can use the balanced chemical equation of the reaction between NaOH and B4O5(OH)4^2- to determine the moles of B4O5(OH)4^2- present in the borax sample.
Finally, we can use the volume of the borax sample and the moles of B4O5(OH)4^2- and Na+ ions to calculate their equilibrium concentrations.
By using the titration data, we can determine the equilibrium concentrations of B4O5(OH)4^2- and Na+ ions in each borax sample solution. These values are important for understanding the behavior and properties of borax in various applications, such as in cleaning agents or as a flux in metallurgy.
To learn more about equilibrium concentration, visit here:
https://brainly.com/question/13414142
#SPJ11
Correct Question:
Use The Titration Data To Calculate The Equilibrium Concentration Of The B4O;(OH)42- And The Na* Ions In Each Borax:

PLEASE HELPPP!!!!! How many grams of sodium are present in a 120 gram sample of
NaHCO3?
Answers
There are approximately 32.89 grams of sodium in a 120-gram sample of NaHCO3.
How to determine the number of moles of NaHCO3 in a 120-gram sampleThe molar mass of NaHCO3 (sodium bicarbonate) can be calculated by adding up the atomic masses of its constituent elements:
NaHCO3 = 1Na + 1H + 1C + 3O
Molar mass of NaHCO3 = 23.0 g/mol + 1.0 g/mol + 12.0 g/mol + (3 × 16.0 g/mol) = 84.0 g/mol
This means that one mole of NaHCO3 has a mass of 84.0 grams.
To determine the number of moles of NaHCO3 in a 120-gram sample, we can use the following equation:
moles = mass / molar massmoles of NaHCO3 = 120 g / 84.0 g/molmoles of NaHCO3 = 1.43 molSince the ratio of Na to NaHCO3 is 1:1, there are also 1.43 moles of Na in the sample.
Finally, we can calculate the mass of sodium (Na) in the sample by multiplying the number of moles of Na by its atomic mass:
mass of Na = moles of Na × atomic mass of Na
mass of Na = 1.43 mol × 23.0 g/mol
mass of Na = 32.89 g
Therefore, there are approximately 32.89 grams of sodium in a 120-gram sample of NaHCO3.
Learn more about molar mass here : brainly.com/question/30459969
#SPJ1
the atmospheric pressure at the summit of mount mckinley is 558 mmhg on a certain day. what is the pressure in atmospheres and in kilopascals? atm kpa
Answers
The atmospheric pressure at the summit of Mount McKinley is 0.736 atm and 98.7 kPa.
To convert the pressure from mmHg to atm, we need to divide it by the standard atmospheric pressure which is 760 mmHg. So, 558 mmHg/760 mmHg = 0.736 atm.
To convert the pressure from atm to kPa, we can use the conversion factor of 1 atm = 101.325 kPa.
Therefore, 0.736 atm x 101.325 kPa/atm = 74.7 kPa (rounded to one decimal place).
Hence, the atmospheric pressure at the summit of Mount McKinley is 0.736 atm and 98.7 kPa.
It is important to note that atmospheric pressure decreases as altitude increases, which is why the pressure at the summit of Mount McKinley is lower than the standard atmospheric pressure at sea level.
Learn more about atmospheric pressure here:
https://brainly.com/question/31634228
#SPJ11
COPY out the following sentences and fill in the gaps.
1. Sulfuric acid releases _____________ ions in solution. This makes the solution _______________________.
2. Sodium hydroxide is an ___________________. It releases ____________________ ions in solution. This makes the solution __________________________.
3. Write word and symbol equations for both of the above situations, a template is given below.
Sulfuric acid --> _____________ + ___________________
________ --> ___________ + ______________
Sodium hydroxide --> _____________________ + ___________________
______________ --> ______________ + __________________
Answers
Answer:
1. Hydrogen ions; acidic
2. Alkali; hydroxide ions; alkaline
3a. Sulfuric acid --> 2 Hydrogen ions + sulfate ion
H₂SO₄ --> 2H+ + SO₄²-
3b. Sodium hydroxide --> Sodium ion + Hydroxide ion
NaOH --> Na+ + OH-
Explanation:
1. Sulfuric acid releases hydrogen ions in solution. This makes the solution acidic.
Acids produce hydrogen ions when dissolved in aqueous solutions.
2. Sodium hydroxide is an alkali. It releases hydroxide ions in solution. This makes the solution alkaline.
Alkalis are soluble bases that produce hydroxide ions in solution.
3a. Sulfuric acid --> 2 Hydrogen ions + sulfate ions
H₂SO₄ --> 2H+ + SO₄²-
The equation above is for the ionization of sulfuric acid
b. Sodium hydroxide --> Sodium ion + Hydroxide ion
NaOH --> Na+ + OH-
The equation above is for the ionization of sodium hydroxide
During decomposition of water,10g of oxygen gas was formed.How much hydrogen gas was formed
Answers
Answer:
https://www.quora.com/The-following-chemical-reaction-shows-the-decomposition-of-water-to-form-hydrogen-gas-and-oxygen-gas-2H2O-I-produces-2H2-g-O2-g-if-10-0-grams-of-water-reacted-and-you-found-1-11-grams-of-H2-formed-how-many-of-O2
Explanation: i hope ths helps you
How many grams of CaCl2 should be dissolved in 445.2 mL of water to make a 0.19 M solution of CaCl2?
Answers
In order to solve this, you will need to know how many moles of CaCl2 you will need, and to do so, you must look at the concentration of the solution.
You are told that the concentration of the solution is 0.19M, meaning that there is 0.19 moles of CaCl2 for every 1 liter of solution. However, you don't have 1 liter of solution, you have 445.2 mL. You must convert this to liters by dividing by 1000.
445.2 mL * (1 L / 1000 mL) = 0.4452 L
Now, you can solve for how many moles the solution must have. Since M = mol / L and you are given M and L, you can set up an equality as follows:
0.19M = mol / 0.4452 L
Now, in order to find the number of moles, you multiply both sides by 0.4452L
0.19M * 0.4452 L = moles = 0.084588mol
Now, you must calculate the molar mass of CaCl2 by using the periodic table
MM Ca = 40.078 g/mol
MM Cl = 35.452 g/mol
40.078 + (35.452 * 2) = 110.982 g/mol
Now you multiply the number of moles in the solution you calculated by the molar mass.
0.084588mol * 110.982 g/mol = 9.387 g CaCl2
Explanation is sorry I forgot the other numbers
A 5.0 milliliter sample of a substance has a mass of 12.5 grams. What is the mass of a 100 milliliter sample of the same substance?
Answers
Answer:
It sould be 250 grams
Explanation:
It is given that, 5 millimeter sample has the mass of 12.5 grams. Then, 100 millimeter sample have a mass of 250 g.
What is mass ?Mass of a substance is a physical quantity that describes the total amount of the substance. Mass is an extensive property since it depends on the change in amount or quantity of the substance.
The SI unit of mass is grams (g). Other units are kilograms, milligrams , pounds etc. Mass is of small substance is expressed in grams and that bigger things are usually expressed in kilograms.
Given that mass of 5 ml sample = 12.5 grams
then mass of 1 ml = 12.5/5 = 2.5 g
then mass of 100 ml = 2.5 × 100 = 250 grams.
Therefore, the mass of 100 ml of the sample is 250 g.
Find more on mass:
https://brainly.com/question/19694949
#SPJ2
A 5.0 l sample of argon gas at 10 oc has a pressure of 760 mm hg. what is the temperature of the gas at 850 mm hg and 6.0 l?
Answers
A 5.0 l sample of argon gas has a pressure of 760 mm hg; the temperature of the argon gas is 24.2°C.
We can use the combined gas law, which states that the pressure, volume, and temperature of a gas are related by the equation PV/T = constant. If we rearrange this equation to solve for temperature, we get T = PV/nR, where n is the number of moles of gas and R is the gas constant.
Since we are dealing with the same sample of gas (argon), we can assume that n and R are constant, and therefore the equation simplifies to T1/T2 = P1V1/P2V2.
Using the given values, we can plug in P1 = 760 mmHg, V1 = 5.0 L, P2 = 850 mmHg, and V2 = 6.0 L. Solving for T2, we get T2 = T1 * P2 * V1 / (P1 * V2) = 10°C * 850 mmHg * 5.0 L / (760 mmHg * 6.0 L) = 24.2°C.
Therefore, the temperature of the argon gas at 850 mmHg and 6.0 L is 24.2°C.
Learn more about combined gas law here:
https://brainly.com/question/30458409
#SPJ11
Convert 92.92 g NaOH to moles
Answers
Answer:
It would be 2.323167848877082
Explanation:
Why are certain elements placed into the same column on the periodic table?
Answers
Answer:
Similar chemical characteristic/behavior
Explanation:
Certain elements placed into the same column on the periodic table, because of a similar chemical characteristic/behavior.
What type of energy does a mosquito get when biting a human
Answers
The energy a mosquito obtains when biting a human is derived from the nutrients present in the blood, which serve as a source of sustenance and facilitate the reproductive cycle of female mosquitoes.
When a mosquito bites a human, it does not directly obtain energy in the form of electricity or any other conventional type of energy. Instead, the mosquito gains sustenance through its feeding behavior. Mosquitoes are hematophagous insects, meaning they feed on the blood of other animals, including humans.
The energy obtained by a mosquito from biting a human comes from the nutrients present in the blood. Mosquitoes have evolved specialized mouthparts that enable them to pierce the skin and access blood vessels. Once they penetrate the skin, they release saliva containing enzymes that prevent blood clotting, allowing them to extract the necessary nutrients.
The primary source of energy for mosquitoes is the sugar found in nectar, which provides them with the fuel required for flight and basic metabolic processes. However, female mosquitoes require additional nutrients, such as proteins and iron, which are crucial for egg development. By biting a human and consuming blood, female mosquitoes acquire these essential nutrients, which contribute to their reproductive capabilities.
For such more questions on nutrients
https://brainly.com/question/13208550
#SPJ8
Make a super hero with the property of Science neutrons
Answers
Answer:
The poster should include:
TITLE: Creative name of superhero.
DESCRIPTION OF ELEMENT SUPERHERO (2 PARAGRAPHS):
This section should include a brief description of how your superhero acquired, used, or lived
with their element power. You may want to describe where does your superhero element lives
(where in nature can it be found). What are his/her superpowers? Does your superhero
element have a hideout? What are his/her strengths? What is his/her weakness? Who is
his/her arch nemesis (evil super villain)? What kind of super suit does your superhero have?
5 FACTS:
This section should include at least 5 fun facts about your element. You can choose to
describe facts that illustrate where your element is found in nature. You may also wish to
describe what is your element used for. You may address what are characteristics of your
element or what are important uses of your element. For example, can your element be made
into something useful or can your element combine with other elements to make something
totally different.)
ATOMIC DRAWING
Include the atomic structure of your element with correct labeling of its protons neutrons,
electrons. Make sure your drawing has the correct number of protons, neutrons, electrons.
DRAWING OF YOUR ELEMENT SUPERHERO
This section should include a drawing (in color) of your Element Superhero on a clean sheet of
WHITE paper. You may want to draw your Element Superhero fighting his nemesis (super
villain). Or you may want to draw your Element Superhero using his special powers.Explanation:
If 250 mL of methane, CH4, effuses through a small hole in 48 s, the time required for the same volume of helium to pass through the hole will be.....?
Answers
If 250 mL of methane (CH4) effuses through a small hole in 48 s, the time required for the same volume of helium to pass through the hole is approximately 96 s.
The effusion rate of a gas is inversely proportional to the square root of its molar mass, according to Graham's law of effusion. In this case, we need to compare the effusion rates of methane and helium.
Since the volume is constant, we can use the ratio of their times of effusion.
Let's assume the molar mass of methane (CH4) is M1 and the molar mass of helium (He) is M2. According to Graham's law, the ratio of the effusion times is given by:
(time for methane) / (time for helium) = √(M2 / M1)
Given that the time for methane is 48 s, we need to find the time for helium. Rearranging the equation, we have:
(time for helium) = (time for methane) / √(M2 / M1)
By substituting the molar masses of methane (16.04 g/mol) and helium (4.00 g/mol), we can calculate:
(time for helium) = 48 s / √(4.00 g/mol / 16.04 g/mol)
(time for helium) = 48 s / √(0.25)
(time for helium) = 48 s / 0.5
(time for helium) = 96 s
Therefore, the time required for the same volume of helium to pass through the hole is approximately 96 seconds.
Learn more about Graham's law of effusion here:
brainly.com/question/30982581
#SPJ11
What type of molecule is pentanal?
A. Aldehyde
O B. Ketone
O C. Alcohol
O D. Ester

Answers
Answer:
\( \huge \color{indigo} \boxed{a. \: aldehyde}\)
Explanation:
Pentanal is considered to be a fatty aldehyde lipid molecule. These are compounds containing more than one aldehyde group. Pentanal is a very hydrophobic molecule, practically insoluble in water, and relatively neutral.
An insoluble solid that forms from a chemical reaction is called
Answers
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary.
Which family (group) of elements has a valence-level electron configuration of 1?
a. noble gases
b. halogens
c. alkali metals
d. alkaline earth metals
Answers
The family (group) of elements that have a valence-level electron configuration of 1 are alkali metals. Hence, the answer is option C.
A group or family in the periodic table consists of all the elements arranged in a single vertical column. There are eighteen groups in total. Elements in the same group tend to have the same chemical properties because they contain the same number of electrons in their outermost shells (valence electrons).
Elements in the first group or group 1 have only one electron in their outermost shell. They are known as alkali metals. They are called alkali metals because they react with water to form bases or alkali. These metals must be stored under oil as they are very reactive and easily react with air and water.
Alkali metals are also very electropositive and form ions by giving out their one valence electron, carrying a charge of +1. The three alkali metals are Lithium (Li), Sodium (Na) and Potassium (K), with Potassium being the most electropositive.
Learn more about alkali metals here:
https://brainly.com/question/18153051
#SPJ4
PLEASE HELP ASAPPP
A cylinder shaped water tank has a height of 212 ft (6461.76 cm), and is able
to hold 15,000 gallons of water (15,000,000 mL or 15,000,000 cm3). The
volume of a cylinder is expressed by the equation
V = tr^2h
Where V is the volume, r is the radius, and h is the height of the cylinder.
Express the radius (r) in terms of volume (V) and height (h), then determine
the radius of a cylinder water tank (in centimeters) that is filled to capacity.
Answers
Answer:
r = \(\sqrt{\frac{v}{\pi h} }\)
27.2cm
Explanation:
Given parameters:
Height of tank = 6461.76cm
Volume of water = 15000000cm³
Volume of cylinder = \(\pi\) r² h
i. Express radius in terms of volume;
we are to make radius the subject of the expression;
v = \(\pi\) r² h
divide both sides by πh
\(\frac{v}{\pi h}\) = \(\frac{\pi r^{2}h }{\pi h }\)
r² = \(\frac{v}{\pi h}\)
r = \(\sqrt{\frac{v}{\pi h} }\)
ii.
Radius of the water tank;
r = \(\sqrt{\frac{15000000}{\pi x 6461.76} }\) = 27.2cm