Based on the following titration curve, what is the concentration of the weak base that is being titrated if the initial volume of base solution is 10 ml and the concentration of the hcl is 0. 2 m?.
Answers
A titration curve is a graph of the pH of a solution versus the volume of the titrant that has been added to it. Titration curves aid in determining the equivalence point of an acid-base reaction, which is when the number of moles of acid equals the number of moles of base.
The equivalence point can be used to determine the concentration of the weak base that is being titrated based on the volume of the titrant used and the concentration of the acid (titrant) used. A weak base is a base that does not completely ionize in water.
They only partially ionize in water to produce hydroxide ions (OH-).To determine the concentration of the weak base that is being titrated based on the given titration curve, we need to identify the equivalence point on the curve. The equivalence point is where the titrant (acid) and the analyte (weak base) are completely reacted. At this point, the pH is at its maximum and is equivalent to the pKa of the weak base.
To know more about curve visit:
https://brainly.com/question/32496411
#SPJ11
Related Questions
The metal zirconium becomes superconducting at temperatures below 3.4000K.
Calculate the temperature at which zirconium becomes superconducting in degrees Celsius. Be sure your answer has the correct number of significant digits.
Answers
Answer:
-269.75 degrees Celsius
Explanation:
hello people ~
(๑¯◡¯๑)what happens when we add carbon tetrachloride in water?
Answers
Answer:
It will explode, I have plenty of research on this. Let me know if you want the sources. :)
Explanation:
Answer:
Explanation:
There will be no reaction when carbon tetrachloride is added to water. This is because the two substances do not mix. It is not due to density difference (like the case of Mercury and water) but rather carbon tetrachloride is non-polar while water is polar. So they will separate and form two different layers with no reaction happening.
73.0mL of nitrogen at STP is heated to 80.0*c and the volume increases to 4.53 L what is the new pressure
Answers
The new pressure of a nitrogen gas at STP is 0.021atm.
How to calculate pressure?The pressure of a gas can be calculated by using the combined gas law equation as follows:
P₁V₁/T₁ = P₂V₂/T₂
Where;
P₁, V₁ and T₁ are the initial pressure, volume and temperature respectivelyP₂, V₂ and T₂ are the final pressure, volume and temperature respectively.At STP, a gas has the following:
P = 1atmT = 273K0.073 × 1/273 = 4.53 × P/353
0.0002674 × 353 = 4.53P
P = 0.021atm
Therefore, 0.021atm is the pressure of the nitrogen gas.
Learn more about pressure at: https://brainly.com/question/10175101
#SPJ1
The new pressure of the gas would be 0.0208 atm.
General gas lawAccording to the general gas law, the ratio of the product of the pressure and volume to its temperature is constant.
The general gas law is summarily expressed as the following equation:
\(p_1v_1/t_1\) = \(p_2v_2/t_2\). where:
\(p_1\) = initial pressure of a gas
\(v_1\) = initial volume of the gas
\(t_1\) = initial temperature of the gas
\(p_2\) = final pressure of the gas
\(v_2\) = final volume of the gas
\(t_2\) = final temperature of the gas
In this case:
\(p_1\) = standard pressure = 1 atm
\(v_1\) = 73 mL or 0.073 L
\(t_1\) = standard temperature = 273 K
\(p_2\) = ?
\(v_2\) = 4.53 L
\(t_2\) = 80 + 273 = 353 K
\(p_2\) = \(p_1v_1t_2/t_1v_2\)
= 1x0.073x353/273x4.53
= 25.769/1236.69
= 0.0208 atm
Thus, the new pressure of 73.0 mL nitrogen that was heated to a volume of 4.53 L at STP is 0.0208 atm.
More on the general gas law can be found here: https://brainly.com/question/28865392
#SPJ1
explanation about reflection in science
Answers
Answer:
the throwing back by a body or surface of light, heat, or sound without absorbing it.
Explanation:
if f(x)=3/x+2 -square root sign x-3
complete the following statement
the domain for f(x) is all real numbers ____ than or equal to 3
Answers
The half-life of the radioactive element unobtanium-31 is 10 seconds. If 176 grams of unobtanium-31 are initially present, how many grams are present after
10 seconds?
20 seconds?
30 seconds?
40 seconds?
50 seconds?
Answers
After 10 seconds, 88 grams of unobtanium-31 are present, after 20 seconds, 44 grams are present, after 30 seconds, 22 grams are present, after 40 seconds, 11 grams are present, and after 50 seconds, 5.5 grams are present.
The amount of unobtanium-31 remaining after certain intervals of time, given that the half-life of the radioactive element is 10 seconds is calculated below.
Initial quantity of the element is given as 176 grams.
After 10 seconds:
Let's first figure out the fraction of the sample remaining after each time period because the half-life of a radioactive element tells us the fraction that decays over a certain period of time.
Using the half-life equation:
amount remaining = original amount x (1/2)^(time elapsed / half-life)
After 10 seconds, the time elapsed is equal to the half-life of the element,
So:
amount remaining = 176 x (1/2)^(10/10)
= 88 grams
After 20 seconds:
amount remaining = 176 x (1/2)^(20/10)
= 44 grams
After 30 seconds:
amount remaining = 176 x (1/2)^(30/10)
= 22 grams
After 40 seconds:
amount remaining = 176 x (1/2)^(40/10)
= 11 grams
After 50 seconds:
amount remaining = 176 x (1/2)^(50/10)
= 5.5 grams
Therefore, after 10 seconds, 88 grams of unobtanium-31 are present, after 20 seconds, 44 grams are present, after 30 seconds, 22 grams are present, after 40 seconds, 11 grams are present, and after 50 seconds, 5.5 grams are present.
Learn more about Radioactive Element from the given link :
https://brainly.com/question/27660984
#SPJ11
SOMEONE HELO PLZ!!!!!

Answers
Answer:
2 8 8 2 is a correct answer
Answer:
2 8 8 2 yup
Explanation:
Someone pls help me I will make you brain

Answers
HELPP ASAP I’ll mark you as brainlister 100 points :((((

Answers
Answer:
the pool table one
Explanation:
cuz gas just goes EVERYWHERE
Which of the following is not a possible sublevel?
a. 1s
b. 2p
c. 3f
d. 4d
Answers
Reason: For principle quantum level 3, there are only s and p orbitals that can exist, you can refer to the four quantum numbers concept to understand this further.
What is the correct voice used when writing an abstract? Select one: First person. Active. Third person. Passive. Second person.
Answers
The common way is the use of active voice when writing an abstract because passive voice make an indirect writing of the action and indication of a person in the action.
What is an abstract ?Abstract is the first phase of a thesis or a publication in a journal. It briefly summarizes about what we are going tp communicate through that paper or thesis what are the major methods used and also tell about the major findings and conclusions.
Thus, abstract must be concise but sharp and clear to the point about each step in the experiment of the scientific investigation whatever. It must be following the same tense of action that is in past or present. All sentence should follow the same tense.
Now, it is commonly better to avoid passive voice to write the actions and if possible write it in active voice. Direct indication of the person in charge and the action is good to read.
Find more on abstract writing:
https://brainly.com/question/14121465
#SPJ1
Which compound forms a powerful acid and also contains a halogen?
A. H2S
B.HBr
C.Li2O
D.LiBr
Answers
Answer:
HBr
Explanation:
Answer:
The answer is in fact HBr
Explanation:
I took the quiz
:)
An insoluble solid that forms from a chemical reaction is called
Answers
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary.
why does stearic acid melt at 69 ∘c but linoleic acid melts at −5∘c?
Answers
The melting points of stearic acid and linoleic acid can be attributed to differences in their molecular structures.
Stearic acid, also known as octadecanoic acid, is a saturated fatty acid with a straight carbon chain consisting of 18 carbon atoms. Each carbon atom in stearic acid is bonded to two hydrogen atoms and a carboxylic acid group (-COOH). The presence of saturated carbon-carbon (C-C) bonds throughout the molecule allows for strong intermolecular interactions, such as van der Waals forces, which require a relatively higher amount of energy to break. As a result, stearic acid has a higher melting point of 69 °C.
On the other hand, linoleic acid is an unsaturated fatty acid with 18 carbon atoms, but it contains two double bonds in its carbon chain. The presence of these double bonds introduces kinks in the molecule, preventing the carbon chain from packing closely together. This reduces the strength of intermolecular forces, resulting in a lower melting point. Linoleic acid has a melting point of approximately -5 °C.
In summary, the difference in the melting points of stearic acid and linoleic acid is primarily due to the presence of saturated versus unsaturated carbon-carbon bonds in their respective molecular structures.
To know more about stearic acid refer here
https://brainly.com/question/925521#
#SPJ11
if a sample has a mass of 127g and a volume of 11mL, what is its density
Answers
Answer:
11.55 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 127 g
volume = 11 mL
We have
\(density = \frac{127}{11} \\ = 11.545454...\)
We have the final answer as
11.55 g/mLHope this helps you
Calculate the mole% of toluene in the vapor mixture at
equilibrium with a liquid mixture of 40% benzene and 60% toluene at
50 oC. Use DIPPR equation.
Answers
The mole percent of toluene in the vapor mixture can be obtained by multiplying y_toluene by 100. substitute the known values into the DIPPR equation, the mole fraction of toluene in the vapor phase (y_toluene) is 47.47%.
To calculate the mole percent of toluene in the vapor mixture at equilibrium with a liquid mixture of benzene and toluene, we can use the DIPPR equation. The DIPPR (Design Institute for Physical Property Data) equation is an empirical equation that relates the vapor-liquid equilibrium (VLE) composition to temperature and composition.
The DIPPR equation for calculating mole fraction in a vapor-liquid equilibrium is given as: y_i = x_i * P_i_sat(T) / P. where: y_i is the mole fraction of component i in the vapor phase, x_i is the mole fraction of component i in the liquid phase, P_i_sat(T) is the vapor pressure of pure component i at temperature T, and P is the total pressure of the system.
In this case, we have a liquid mixture of 40% benzene and 60% toluene. Let's assume a total pressure of P for the system and consider the vapor phase at equilibrium. We can use the DIPPR equation to calculate the mole fraction of toluene (y_toluene) in the vapor phase.
First, we need to determine the mole fraction of toluene in the liquid phase (x_toluene). Since the liquid mixture is composed of 40% benzene and 60% toluene, we have x_toluene = 0.60. Next, we need the vapor pressure of pure toluene at the given temperature of 50 °C. We can obtain this value from reliable sources or thermodynamic databases, such as the DIPPR database.
Finally, substitute the known values into the DIPPR equation to calculate the mole fraction of toluene in the vapor phase (y_toluene). The mole percent of toluene in the vapor mixture can be obtained by multiplying y_toluene by 100.
It's important to note that the DIPPR equation is an approximation, and for accurate calculations, it's advisable to consult more comprehensive thermodynamic models or databases specific to the system being analyzed.
We can calculate the mole fraction of toluene in the vapor phase: yT = 0.6 * 0.7911 = 0.4747 Therefore, the mole% of toluene in the vapor mixture at equilibrium with a liquid mixture of 40% benzene and 60% toluene at 50°C is 47.47%.
To know more about vapor mixture refer:
https://brainly.in/question/32733739
#SPJ11
which is more stable: 10 protons, 12 neutrons, and 10 electrons when they are combined as two 11 b atoms or as one 22 ne atom?
Answers
Both combinations are stable, but the stability of atoms is determined by the balance of the attractive forces of protons and the repulsive forces between protons in the nucleus, as well as the balance of attractive forces between electrons and the positively charged nucleus.
In this case, the combination of 10 protons and 12 neutrons can form two different isotopes of boron: 11B (with 1 neutron) and 11B (with 2 neutrons). Both of these isotopes are stable, although 11B is more abundant.
On the other hand, the combination of 10 protons and 12 neutrons can also form a stable neon isotope, 22Ne, which has 10 electrons in its neutral state.
In terms of stability, both combinations are energetically favorable and stable, and it is not possible to say which is more stable without further information.
Learn more about protons here:
https://brainly.com/question/30895149
#SPJ11
As an animal grows, its mass increases. The matter that makes up this new mass comes mostly from
Answers
As an animal grows, its mass increases. The matter that makes up this new mass comes mostly from the food that the animal consumes.
What is matter?Matter in chemistry, is defined as any kind of substance that has mass and occupies space that means it has volume .Matter is composed up of atoms which may or not be of same type.
Atoms are further made up of sub atomic particles which are the protons ,neutrons and the electrons .The matter can exist in various states such as solids, liquids and gases depending on the conditions of temperature and pressure.
The states of matter are inter convertible into each other by changing the parameters of temperature and pressure.
Learn more about matter,here:
https://brainly.com/question/9477180
#SPJ1
SOMEONE PLEASE HELP HURRY

Answers
I think the answer is A. I know it's Planet X.
Brainliest to first decent answer
What is the chemical formula for the molecule represented by the model?
CHO
C4H9O2
C4H8O
C3H8O2

Answers
The correct formula of the molecule is C4H9O2.
What is a model?The model of a compound is a representation of the molecule. It gives us an idea of what the molecule looks like as well as its molecular formula.
Looking at the structure of the compound as shown in the model in the question, the correct formula of the molecule is C4H9O2.
Learn more about molecular model:https://brainly.com/question/156574?
#SPJ1
Answer:C4H9O2.
Explanation:
Decide which intermolecular forces act between the molecules of each compound in the table below.
Answers
Answer
• Hydrogen bromide: dispersion and dipole-dipole
,• Bromine: dispersion
,• Hypochlorus acid : dispersion, dipole-dipole, hydrogen bond
,• Molecular oxygen: dispersion
Procedure
The London dispersion force is a temporary attractive force that results when the electrons are in two adjacent atoms. These are the weakest intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar.
Dipole-dipole: occur between polar molecules when the partially positively charged part of a molecule interacts with the partially negatively charged part of another molecule.
A hydrogen bond: is a special kind of dipole-dipole interaction that occurs specifically between a hydrogen atom bonded to an oxygen, nitrogen, or fluorine atom.
Based on the previous:
Hydrogen bromide: dispersion and dipole-dipole
Bromine: dispersion
Hypochlorus acid : dispersion, dipole-dipole, hydrogen bond
Oxygen: dispersion
when does the system in the graph reach equilibrium? pleaseeee help fast!!
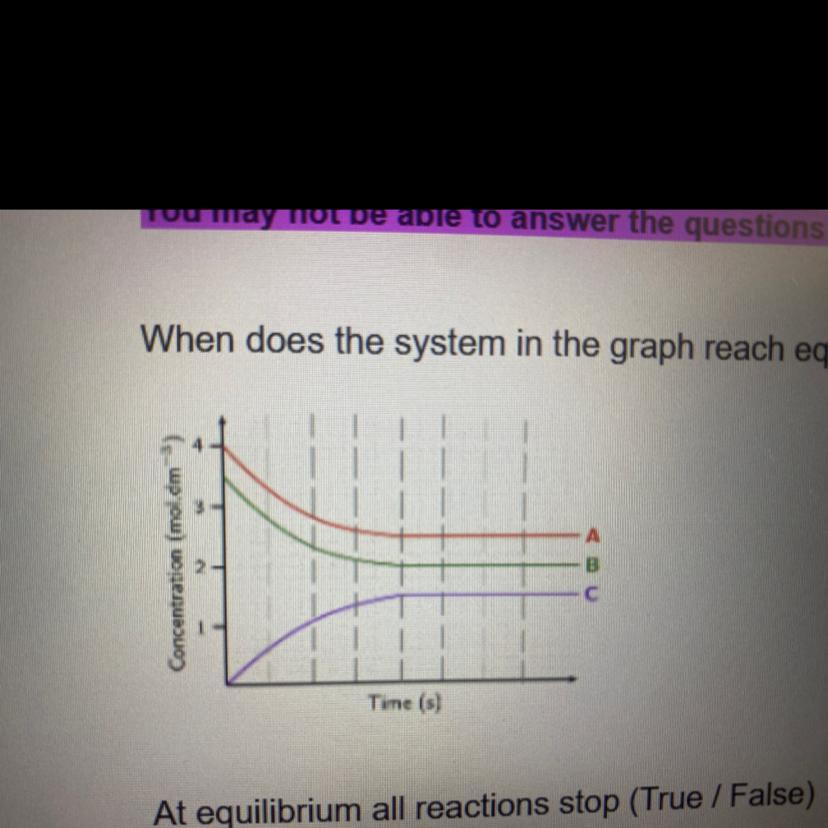
Answers
The system is now in equilibrium when both the forward reaction rate and the reverse reaction rate are equal. When the concentration and number of moles of a products and reactants are constant.
How does an ecological system come to balance?Whenever the rates of the forward or reverse reactions are equal, a system is said to be in equilibrium. The pace of the ahead reaction rises with the addition of more reactants. It appears that the equilibrium is shifting towards the product, and right, part of the equation since the rate of a reverse reaction remains unchanged at first.
How can equilibrium be determined?When there is no trend for the amounts of reactants and byproducts to fluctuate, a chemical process is in equilibrium. They both produce the same mixture all components after the transition is complete, and they both represent the very same chemical reaction process in which the components' functions are reversed.
To know more about moles visit:
https://brainly.com/question/26416088
#SPJ1
Describe and provide detailed mechanism (use arrow pushing) for the preparation of 1,2- dibromo-1,2-diphenylethane 2 pts Provide potential undesired (side) reaction that can occur during the preparation of the 1,2- dibromo-1,2-diphenylethane_.
Answers
1,2-dibromo-1,2-diphenylethane is prepared through the bromination of trans-stilbene, a reaction involving an electrophilic addition mechanism.
The reaction starts with the generation of a bromine radical (Br•) by a free-radical initiator. This radical reacts with trans-stilbene, producing a brominated stilbene radical (Ph-CH=CH-Ph•Br). The brominated radical further reacts with another bromine radical to form the final product, 1,2-dibromo-1,2-diphenylethane (Ph-CHBr-CHBr-Ph).
Arrow pushing in the mechanism:
1. The π bond of trans-stilbene donates an electron pair to Br•, forming a bond between the carbon and bromine.
2. The brominated stilbene radical donates an electron pair to another Br•, forming a bond between the second carbon and bromine.
A potential undesired side reaction is the formation of 1,1-dibromo-1,2-diphenylethane, a regioisomer. This occurs when the brominated stilbene radical reacts with another bromine molecule (Br₂) instead of a bromine radical. The carbon-bromine bond in the intermediate species can break, forming a carbocation (Ph-CHBr-CH⁺-Ph) and a bromide ion (Br⁻). The carbocation then captures the bromide ion, resulting in the undesired product (Ph-CHBr₂-CHBr-Ph).
Arrow pushing in the side reaction:
1. The brominated stilbene radical donates an electron pair to Br₂, forming a bond between the second carbon and one bromine.
2. The carbon-bromine bond in the intermediate species breaks, producing a carbocation and a bromide ion.
3. The carbocation captures the bromide ion, forming the undesired product.
To know more about bromination, refer to the link below:
https://brainly.com/question/14678883#
#SPJ11
Due to risk of electrical shock, team member must be careful not to let any water splash into lemonade dispenser while panels are off for cleaning.
a. true
b. false
Answers
b. false Water is a good conductor of electricity, and if it comes into contact with electrical components or wiring, it can create a risk of electrical shock. Therefore, team members should indeed be careful not to let water splash into the lemonade dispenser when the panels are off for cleaning.
This precaution is necessary to ensure the safety of the individuals handling the equipment. It is important to note that electrical shock can occur when there is a complete or partial path for electric current to flow through the body. Water can provide this path of conductivity and increase the likelihood of electrical shock. Therefore, team members should take appropriate precautions to prevent water from reaching the electrical components and ensure their own safety.
Learn more about panels here: brainly.com/question/6949231
#SPJ11
Mole Calculation Worksheet. Answer the following questions: 1) How many moles are in 25.0 grams of water, H₂O? What is the percent composition for each element? 2) How many grams are in 4.500 moles of Li₂O? What is the percent composition for each element? 3) How many molecules are in 23.0 moles of oxygen, O₂? What is the percent composition for each element? 4) How many moles are in 3.4 x 10¹ molecules of H₂SO,? What is the percent composition for each element? 5) How many molecules are in 25,0 grams of NH,? What is the percent composition for each element?
Answers
There are approximately 1.387 moles in 25 grams of water (1) and 134.46 grams in 4.5 moles of Li₂O (2).
1. To calculate the number of moles, we need to divide the given mass of water by the molar mass of water, which is approximately 18.015 g/mol.
Number of moles = 25 grams / 18.015 g/mol
≈ 1.387 moles
Therefore, there are approximately 1.387 moles in 25 grams of water.
2. To calculate the mass in grams, we need to multiply the number of moles by the molar mass of Li₂O, which is approximately 29.88 g/mol.
Mass in grams = 4.5 moles x 29.88 g/mol
≈ 134.46 grams
Therefore, there are approximately 134.46 grams in 4.5 moles of Li₂O.
3. To determine the number of molecules in 23 moles of oxygen, we can use Avogadro's number, which states that there are 6.022 x 10²³ molecules in one mole of any substance. Therefore, for 23 moles of oxygen, we can calculate:
Number of molecules = 23 moles x 6.022 x 10²³ molecules/mole
= 1.38646 x 10²⁵ molecules
So, there are approximately 1.38646 x 10²⁵ molecules in 23 moles of oxygen.
4. To determine the number of moles in 3.4 x 10²³ molecules of H₂SO₄, we can use Avogadro's number. Since one mole contains 6.022 x 10²³ molecules, we can calculate:
Number of moles = (3.4 x 10²³ molecules) / (6.022 x 10²³ molecules/mole)
= 0.564 moles
So, there are approximately 0.564 moles in 3.4 x 10²³ molecules of H₂SO₄.
5. To determine the number of molecules in 25 grams of NH₃, we need to convert grams to moles using the molar mass of NH₃. The molar mass of NH₃ is 17.03 g/mol.
Number of moles = 25 grams / 17.03 g/mol
≈ 1.468 moles
Using Avogadro's number, we can calculate the number of molecules:
Number of molecules = (1.468 moles) x (6.022 x 10²³ molecules/mole)
= 8.831 x 10²³ molecules
So, there are approximately 8.831 x 10²³ molecules in 25 grams of NH₃.
The complete question is:
Answer the following questions:
1) How many moles are in 25 grams of water?
2) How many grams are in 4.5 moles of Li₂O?
3) How many molecules are in 23 moles of oxygen?
4) How many moles are in 3.4 x 10²³ molecules of H₂SO₄?
5) How many molecules are in 25 grams of NH₃ ?
To know more about moles follow the link:
https://brainly.com/question/15209553
#SPJ4
This is the process that cells reproduce and replace old or damaged cells.
Question 5 options:
Mitosis
Meiosis
Ribosomes
Cytokinesis
Answers
Didnndxbdbdn
what is enviorement.
Answers
Answer:
the natural and man made thing which we can see around us is known as environment. not enviorement.
Answer:
Environment means anything that surrounds us. It can be living (biotic) or non-living (abiotic) things. It includes physical, chemical and other natural forces. ... In the environment there are different interactions between animals, plants, soil, water, and other living and non-living things.
What mass of K2SO4 must be added to 1.20 liters of water to produce a 1.50 M solution?
Answers
Answer:
313.2 g of \({K_{2} }S O_{4}\) must be added to 1.20 liters of water to produce a 1.50 molar solution.
Explanation:
What is molarity?
Molarity is a unit of concentration of a solution. It is defined by the number of moles of the solute that is present in one liter (1L) of the solution. It is denoted by M. Thus, molarity = \(\frac{Number of moles of the solute (n) }{Volume of the solution (V) (in L)}\)∴ The number of moles of solute = molarity x volume of the solution.According to the given question,
Molarity of the solution = 1.50 MThe volume of the solution = 1.20 LUnknown = Mass of \({K_{2} }S O_{4}\) required.Solution :
∴ Number of moles of solute, here, \({K_{2} }S O_{4}\)
= molarity x volume of the solution
= 1.20 x 1.50 = 1.8
∴ Mass of 1.8 moles of \({K_{2} }S O_{4}\) = 1.8 x molar mass of \({K_{2} }S O_{4}\)
Now the molar mass of \(K_{2} SO_{4}\)
= (Gram atomic mass of K x 2) + (Gram atomic mass of S) + (Gram atomic mass of O x 4)
= (39x2) + 32 + (16 x 4) g
= 174 g.
∴ Mass of 1.5 moles of \({K_{2} }S O_{4}\)
= 1.8 x molar mass of \({K_{2} }S O_{4}\)
= 1.8 x 174 g
= 313.2 g.
Thus, 313.2 g of \({K_{2} }S O_{4}\) must be added to 1.20 liters of water to produce a 1.50 M solution.
To know more about molarity, visit :
https://brainly.com/question/15406534
Why are small quantities of chlorofluorocarbons so harmful to the ozone layer? a. The chlorofluorocarbons act like ultraviolet radiation causing large amount of ozone to be produced. b. The chlorine from the chlorofluorocarbons reacts with free molecules of oxygen causing a stop in ozone production. c. Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone. d. Chlorofluorocarbons absorb ultraviolet radiation, preventing the formation of ozone.
Answers
Answer:
Why are small quantities of chlorofluorocarbons so harmful to the ozone layer? Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone.
Explanation:
The statement for small quantities of chlorofluorocarbons so harmful to the ozone layer is "Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone."
What is ozone layer?The ozone layer is a thin layer of air in the Earth's atmosphere that absorbs nearly all of the sun's damaging UV radiation.
What is CFCs?CFCs (chlorofluorocarbons) are harmless and nonflammable compounds made up of carbon, chlorine, and fluorine atoms.
The earth's protective ozone layer is destroyed by chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and halons, which shield the earth from damaging ultraviolet (UV-B) rays released by the sun. CFCs and HCFCs also warm the earth's lower atmosphere, causing global climate change.
When some substances are exposed to high UV radiation in the stratosphere, they emit chlorine or bromine. Ozone-depleting chemicals are compounds that contribute to ozone depletion (ODS). Chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform are examples of ODS that produce chlorine. Halons and methyl bromide are two ODS that emit bromine.
Because there isn't much ozone in the atmosphere, what little there is is critical for protecting the Earth's surface from excessive UV light from the Sun. It turns out that it reacts with chlorine, which means that chlorine effectively eliminates ozone.
When the chlorine in CFCs combines with ultraviolet light, it releases chlorine, which then reacts with ozone, reducing the protection humans get from ultraviolet light, allowing more CFCs to release chlorine, and so on. Multiple ozone molecules will interact with one free chlorine atom, which is free because UV light has hit the CFC molecule. As a result, the damage it can cause is likely to be significantly more than you might imagine.
Hence the correct option is c.
Learn more about ozone layer and CFCs here
https://brainly.com/question/14330630
#SPJ2
Select the correct answer. Given: 2LiBr Ba → BaBr2 2Li In this chemical reaction, 325 grams of barium (Ba) react completely. How many moles of lithium (Li) are produced? A. 1. 18 mol B. 2. 37 mol C. 4. 73 mol D. 16. 4 mol E. 32. 9 mol.