barium chloride (bacl2) emits a green color when flame-tested. what can be said about the wavelength of light it emits?
Answers
Barium chloride emits a green color when examined using a flame. This means that heat energy is being transferred to the barium ions' outer electrons.
A substance emits visible light when its electrons transition from a higher energy level to a lower energy level. This is due to the electrons' ability to emit energy when transferring in a lower region.
This is only conceivable when the electrons are excited to a higher energy level with sufficient energy.
In addition, when the electrons return to their ground state, they release energy that emits a green light with a wavelength of 500–560 nm.
To learn more about Barium chloride:
https://brainly.com/question/24112864?referrer=searchResults
#SPJ4
Related Questions
Sodium Acetate 4 (g) what is molarity of sodium acetate (m)
Answers
The final answer will be the molarity of sodium acetate in moles per liter (M). Molarity is a unit of concentration that represents the number of moles of solute (in this case, sodium acetate) per liter of solution.
Without knowing the molarity of the solution or the amount of solvent (usually water) used to dissolve the sodium-acetate, it is impossible to determine the molarity of the solution. To find the molarity of a solution, you need to know the number of moles of solute and the volume of the solution in liters. Here is a three-paragraph explanation of how to calculate molarity: First, you need to determine the number of moles of solute in the solution. This can be done using the formula: moles = mass / molar mass
The molar mass of sodium acetate is 82.03 g/mol, so if you have 4 g of sodium acetate, the number of moles would be:
moles = 4 g / 82.03 g/mol = 0.0488 moles
Next, you need to determine the volume of the solution in liters. This can be done by measuring the volume directly if you have a graduated cylinder or pipette, or by calculating the volume using the formula: volume = mass / density
If you know the mass of the solution and its density, you can use this formula to find the volume in liters. Finally, you can calculate the molarity of the solution using the formula: molarity = moles / volume
By plugging in the values for moles and volume that you have determined, you can calculate the molarity of the sodium acetate solution. To find the molarity of sodium acetate, you'll need three key pieces of information: the mass of sodium acetate, the molecular weight of sodium acetate, and the volume of the solution.
To know more about molarity visit :-
https://brainly.com/question/23686981
#SPJ11
I need answers. Thanks in advance
Answers
what do you need answers too ?
What brand of canned tuna can a person with ckd that is low in soium, potassium and phosphorus?
Answers
A person with chronic kidney disease (CKD) who needs a low-sodium, low-potassium, and low-phosphorus canned tuna can consider brands that offer "no salt added" or "low sodium" options. One example of a brand that provides such options is "Safe Catch."
Safe Catch offers canned tuna products that are specifically designed to be low in sodium, potassium, and phosphorus. They have a "no salt added" variety that contains minimal sodium, making it suitable for individuals with CKD who need to restrict their sodium intake. Additionally, their products are tested for mercury and other contaminants, providing an extra level of safety.
It is important for individuals with CKD to carefully read the labels and nutritional information of canned tuna products to ensure they meet their specific dietary needs.
Look for brands that explicitly state low sodium or no salt added to ensure minimal sodium content. Furthermore, consulting with a healthcare professional or a registered dietitian who specializes in renal nutrition can provide personalized recommendations based on individual dietary requirements and restrictions.
Learn more about chronic kidney visit:
https://brainly.com/question/29356731
#SPJ11
_____ is responsible for bringing gravity into modern science.
Isaac Newton
Galileo Galilei
Albert Einstein
Answers
The theory of what an atom looks like has evolved over time. From the ancient Greeks to Dalton, to Rutherford—no idea was exactly the same. However, as more information is gathered, new ideas can build on the old. Sometimes new ideas can completely replace the old ones. Even today, there may be new advances that have changed our understanding of the atom that is not reflected in this course.
Answer one of the following prompts to begin your discussion:
You claim that the atomic model should not be continually changed. What reasoning would you give someone to help them understand your claim?
You claim that a new atomic model should always build on an old one. What reasoning would you give someone to help them understand your claim?
You claim that a new atomic model should completely replace an old one. What reasoning would you give someone to help them understand your claim?
You claim that the continuous evolution of the atomic model is beneficial, but you think it should be a mix of the old and the new. What reasoning would you give someone to help them understand your claim?
Answers
A solution of NaOH had a concentration of 20 g/dm3 What mass of NaOH would there be in 250 cm3 of the solution?
Answers
Answer:
5g NaOH
Explanation:

Use The Periodic Table To Determine The Number Of 2p Electrons In F. Number Of 2p Electrons: Use The Periodic Table To Determine The Number Of 3p Electrons In Si, Number Of 3p Electrons: Use The Periodic Table To Determine The Number Of 3d Electrons In Fe. Number Of 3d Electrons: Use The Periodic Table To Determine The Number Of Ap Electrons In Kr. Number Of
Answers
Using the periodic table the number of electrons is determined as;
Fluorine (F) has 5 electrons in 2p orbital.
Silicon (Si) has 2 electrons in 3p orbital.
Iron (Fe) has 6 electrons in 3d orbital.
Krypton (Kr) has 6 electrons in 4p orbital.
The number of electrons in each orbital or the electronic configuration can be determined by the Aufbau principle, along with other principles such as the Pauli exclusion principle and Hund's rule.
1. The atomic number of Fluorine is 9, which means it has 9 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, and 5 electrons are in the 2p orbital. Therefore, the number of 2p electrons in F is 5.
2. The atomic number of Silicon is 14, which means it has 14 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, 6 electrons are in the 2p orbital, and 2 electrons are in the 3s orbital. Therefore, the number of 3p electrons in Si is 2.
3. The atomic number of Iron is 26, which means it has 26 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, 6 electrons are in the 2p orbital, 2 electrons are in the 3s orbital, and 6 electrons are in the 3p orbital. Therefore, the number of 3d electrons in Fe is 6.
4. The atomic number of Krypton is 36, which means it has 36 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, 6 electrons are in the 2p orbital, 2 electrons are in the 3s orbital, 6 electrons are in the 3p orbital, 10 electrons are in the 3d orbital, and 2 electrons are in the 4s orbital. Therefore, the number of 4p electrons in Kr is 6.
Learn more about electronic configuration: https://brainly.com/question/26084288
#SPJ11
I NEED HELP, THANKS!
Using the Ideal Gas Law, PV = nRT, where R = 0.0821 L atm/mol K, calculate the volume in liters of oxygen produced by the catalytic decomposition of 25.5 g potassium chlorate according to the following reaction. The oxygen is collected at 2.22 atm and 25.44°C. Express your answer to the correct number of significant figures.

Answers
Answer:
\(\large \boxed{\text{3.45 L}}\)
Explanation:
We will need a balanced chemical equation with molar masses, so, let's gather all the information in one place.
Mᵣ: 122.55
2KClO₃ ⟶ 2KCl + 3O₂
m/g: 25.5
(a) Moles of KClO₃
\(\text{Moles of KClO}_{3} =\text{25.5 g KClO}_{3} \times \dfrac{\text{1 mol KClO}_{3}}{\text{122.55 g KClO}_{3}} = \text{0.2081 mol KClO}_{3}\)
(b) Moles of O₂
The molar ratio is 3 mol O₂:2 mol KClO₃
\(\text{Moles of O$_{2}$}= \text{0.2081 mol KClO}_{3} \times \dfrac{\text{3 mol O$_{2}$}}{ \text{2 mol KClO}_{3}} = \text{0.3121 mol O$_{2}$}\)
(c) Volume of O₂
We can use the Ideal Gas Law to calculate the volume of hydrogen.
pV = nRT
T = (25.44 + 273.15) K = 298.59 K
\(\rm V = \dfrac{nRT}{p}= \dfrac{\text{0.3121 mol $\times$ 0.0821 L$\cdot$atm$\cdot$K$^{-1}$mol$^{-1}\times$ 298.59 K}}{\text{ 2.22 atm}} = \textbf{3.45 L} \\\\\text{The volume of oxygen is $\large \boxed{\textbf{3.45 L}}$}\)
Answer:
Solution:-
The balanced equation:
2KClO3 (s) \rightarrow 2KCl (s) + 3 O2 (g)
Molar mass of KClO3 = 122.55 g/mol
Number of moles of KClO3 = (Mass of KClO3 / Molar mass of KClO3) = (25.5 g / 122.55 g/mol) = 0.2081 mol
From balanced equation, 2 mol of KClO3 produce 3 mol of O2. Or, 1 mol of KClO3 produces (3/2) mol of O2.
therefore, 0.2081 mol of KClO3 will produce = (3/2) × (0.2081) = 0.3121 mol of O2
Now, we have number of moles (n) of O2 = 0.3121 mol
Pressure (P) = 2.22 atm
Temperature (T) = 25.44°C = (273.15 + 25.44) K = 298.59 K
R (Gas constant) = 0.0821 L.atm/mol.K
Volume (V) of O2 = ?
Using the ideal gas equation,
V = nRT / P
Substituting the values in the equation, we get :
V = (0.3121 mol × 0.0821 L.atm/mol.K × 298.59 K) / (2.22 atm) = 3.45 L
Hence, the volume of O2 gas produced = 3.45 L
Explanation:

When carbon disulfide, CS2, forms from its elements. Heat is absorbed. How much heat would be required to produce 5.0 moles of carbon disulfide
Answers
Answer:
5.9 × 10² kJ
Explanation:
When carbon disulfide, CS₂, forms from its elements, heat is absorbed. The corresponding value for the standard enthalpy of formation of carbon disulfide is 117.36 kJ/mol. The thermochemical equation that represents this process is:
C(graphite) + 2 S(s, rhombic) ⇒ CS₂(g) ΔH°f = 117.36 kJ/mol
117.36 kJ of heat are absorbed when 1 mole of CS₂ is formed. The amount of heat absorbed when 5.0 moles of CS₂ are formed is:
5.0 mol × 117.36 kJ/mol = 5.9 × 10² kJ
Sodium chloride is dissolved in water.
Which ions are present in the electrolyte?
Answers
Answer:
Na+
Cl-
H+
OH-
Explanation:
NaCl + H2O -----> Na+. Cl- + H+ OH-
An element has an atomic number 17.How many electrons are present in K,L and M shells of the atom?
Answers
Answer:
k=2, L=8, M=7
Explanation:
because k shell have maximum electron number of 2 ,L shell and M shell have 8 and 18 respectively.
Determina el grado de pureza de un marmol (CaCO3), si al descomponerse 125 g del mismo se desprenden 20 litros de dióxido de carbono medidos a 15ºC y 1 atm.
Answers
Answer:
67.8%
Explanation:
La reacción de descomposición del CaCO₃ es:
CaCO₃ → CO₂ + CaO
Donde 1 mol de CaCO₃ al descomponerse produce 1 mol de CO₂ y 1 mol de CaO.
Usando la ley general de los gases, las moles de dioxido de carbono son:
PV = nRT.
Donde P es presión (1atm), V es volumen (20L), n son moles de gas, R es la constante de los gases (0.082atmL/molK) y T es temperatura absoluta (15 + 273.15 = 288.15K). Reemplazando los valores en la ecuación:
PV / RT = n
1atmₓ20L / 0.082atmL/molKₓ288.15K = 0.846 moles
Como 1 mol de CO₂ es producido desde 1 mol de CaCO₃, las moles iniciales de CaCO₃ son 0.846moles.
La masa molar de CaCO₃ es 100.087g/mol. Así, la masa de 0.846moles de CaCO₃ es:
0.846moles ₓ (100.087g / mol) = 84.7g de CaCO₃
Así, la pureza del marmol es:
(84.7g de CaCO₃ / 125g) ₓ 100 =
67.8%for the following battery: Cd(s)line CdCl2(aq)double line Cl^-(aq) line Cl2(l) line C(s)
A) Write the reduction half reaction occuring at the C(s) electrode . (Include physical states of reactants and products.)
C(s) electrode: please provide.
E^*=1.4 V
B) From which electrode will electrons flow from the battery into a circuit.
1. Cd(s) electrode
2.C(s) electrode
C) calculate the mass of Cl2 consumed if the battery delivers a constant current of 713 A for 30.0 min.
Answer must be in Kg.
Answers
A) The reduction half reaction occurring at the C(s) electrode is: C(s) + 2Cl^-(aq) -> Cl2(l) + 2e^-. B) Electrons will flow from the Cd(s) electrode into the circuit.
C) To calculate the mass of Cl2 consumed, we need to first calculate the total charge passed through the circuit using the formula:
charge = current x time
charge = 713 A x 30.0 min x 60 s/min
charge = 1,284,000 C
We can then use Faraday's constant to convert the charge to moles of electrons:
1 F = 96,485 C/mol
mol e^- = charge / (1 F)
mol e^- = 1,284,000 C / (1 F)
mol e^- = 13.31 mol
From the balanced half reaction, we know that 1 mole of electrons is needed to produce 1 mole of Cl2. Therefore, 13.31 mol of Cl2 is produced. We can convert this to mass using the molar mass of Cl2:
mass = moles x molar mass
mass = 13.31 mol x 70.906 g/mol
mass = 944.4 g = 0.9444 kg (to three significant figures).
To learn more about reaction visit;
https://brainly.com/question/30464598
#SPJ11
What does mean when it asks for the smaller ion
Answers
Explanation:
mg2+ would be the smaller ion this is because each ion has the same number of electrons however mg2+ has a greater number of protons and therefore is more charge dense and the outer electrons feel a greater pull from the nucleus.
I hope it's helpful!!
which is more concentrated 200 ML of an 8-M NaOH solution or 500 mL of a 4-M NaOH solution
Answers
Answer:
200 ML of an 8-M NaOH solution; The higher the molarity, the higher the concentration of the substance. The volume of the 8M NaOH solution is less as well.
Molarity of a solution defines the concentration of a solution. It is the amount of the solute in 1 L of water. Mathematically it is expressed as:
\(Molarity = \frac{Mole}{Volume}\)
From the formula above, the molarity and the volume are in inverse proportionality.
Thus, an increase in the volume of water will leads to a decrease in the concentration while a decrease in the volume of water will lead to an increase in the concentration.
We shall compare both solutions as follow:
Solution 1:Volume = 200 mL
Molarity = 8 M
Solution 2:Volume = 500 mL
Molarity = 4 M
We can see that solution 1 has a lower volume than solution 2. Hence, the concentration of solution 1 is more than that of solution 2.
From the illustrations above, we can conclude that 200 mL of a 8M NaOH solution is more concentrated than 500 mL of a 4M NaOH solution.
Learn more: https://brainly.com/question/16587536
a solution containing 20.0 g of an unknown non-electrolyte liquid and 110.0 g water has a freezing point of -1.32 °c. given kf = 1.86°c/m for water, the molar mass of the unknown liquid is ____g/mol.
Answers
The molar mass of the unknown non-electrolyte liquid is given as
256 g/mol, option A.
The ratio between the mass and the amount of substance (measured in moles) of any sample of a chemical compound is known as the molar mass (M) in chemistry. The molar mass of a material is a bulk attribute rather than a molecular one.
ΔTemp.f = i x Kf x b
where,
ΔTemp.f = the freezing-point depression;
i = the Van't Hoff factor
Kf = the cryoscopic constant of the solvent;
b = the molality of the solution.
Solving for the molality, b = ΔTemp.f/( i * Kf)= 1.32/(1*1.86)
= 0.71 mol/kg
Converting from mol/kg to mol/g,0.71 mol/kg * 1kg/1000g
= 0.00071 mol/g.
Mass of solvent = 110gNumber of moles = mass * molality
= 0.00071 * 110
= 0.078 mol.
To calculate molar mass,Molar mass (g/mol) = mass/number of moles
Mass of solute (liquid) = 20g
Molar mass = 20/0.078
= 256.2 g/mol. ≈ 256 g/mol
Therefore, molar mass of the unknown liquid is 256.2 g/mol.
Learn more about Molar mass:
https://brainly.com/question/837939
#SPJ4
Complete question:
A solution containing 20.0 g of an unknown non-electrolyte liquid and 110.0 g water has a freezing point of -1.32 °c. given kf = 1.86°c/m for water, the molar mass of the unknown liquid is ____g/mol.
A)256B) 69.0 C) 619 D) 78.1
The molar mass of the unknown liquid is 256.5 g/mol.To solve this problem, we can use the formula for calculating the freezing point depression: ΔTf = Kf·m·i
where ΔTf is the change in freezing point (in °C), Kf is the freezing point depression constant (in °C/m), m is the molality of the solution (in mol/kg), and i is the van't Hoff factor (which is 1 for non-electrolytes).
First, we need to calculate the molality of the solution:
molality = moles of solute / mass of solvent (in kg)
We know that the mass of the solvent (water) is 110.0 g, which is 0.1100 kg. To find the moles of solute (the unknown liquid), we need to divide its mass (20.0 g) by its molar mass (which we don't know yet). Let's call the molar mass "M":
moles of solute = 20.0 g / M
So, the molality is:
molality = (20.0 g / M) / 0.1100 kg
molality = (20.0 / M) / 0.1100 mol/kg
Now, we can plug this into the formula for freezing point depression:
ΔTf = Kf·m·i
-1.32 = 1.86·[(20.0 / M) / 0.1100]·1
Simplifying this equation, we get:
-1.32 = 1.86·(181.8 / M)
-1.32 = 338.628 / M
M = 338.628 / 1.32
M = 256.5 g/mol
Therefore, the molar mass of the unknown liquid is 256.5 g/mol.
Learn more about freezing point here: brainly.com/question/3121416
#SPJ11
You are cooking dinner using a metal pan. When you pick up the pan,the handle burns your hand. Which form of thermal energry transfer causes you to burn your hand?
a conduction
b convection
c radiation
Answers
Answer:
Conduction
Explanation:
Answer: The answer would be A conduction
what type of radiation corresponds to a wavelength of 2.86 X 10 -8m?wha one would it beGammaUltravioletInfraredRadio
Answers
Answer
Ultraviolet
Explanation
The types of radiation and their wavelength are:
Gamma < 10⁻¹² m
X-rays 1 nm - 1 pm
Ultraviolet 400 nm - 1 nm
Visible 750 nm - 400 nm
Near-infrared 2.5 μm - 750 nm
Infrared 25 μm - 2.5 μm
Microwaves 1 mm - 25 μm
Radio waves > 1 mm
Hence, the given wavelength of 2.86 X 10⁻⁸ m corresponds to Ultraviolet.
Why does electron affinity tend to become more exothermic as you move right across a period? a. The trend in electron affinity is just the opposite of that for first ionization energy, since the two processes are the oppose of each other. b. As you move right across the period, you have a greater likelihood of pairing electrons in an orbital, which is a more stable configuration. c. As you move right across the period, metallic character decreases, meaning that the atom or ion is more likely to gain electrons than lose electrons. d. The effective nuclear charge increases as you move right across the period, resulting in greater attraction for an electron.
Answers
Electron affinity tend to become more exothermic as you move right across a period because the effective nuclear charge increases as you move right across the period, resulting in greater attraction for an electron. The correct answer is: D.
Electron affinity is the energy change that occurs when an electron is added to a neutral atom in the gaseous state. It is measured in kJ/mol and is typically reported as a positive or negative value.
A positive electron affinity means that energy is required to add an electron to the atom, while a negative electron affinity means that energy is released when an electron is added to the atom.
The effective nuclear charge is the net positive charge experienced by an electron in an atom. It is calculated by subtracting the number of shielding electrons from the number of protons in the nucleus.
As you move right across a period, the effective nuclear charge increases because each successive element has one more proton in its nucleus.
This increase in effective nuclear charge results in a greater attraction for an electron, which makes it more likely for an atom to gain an electron.
Therefore, electron affinity tends to become more exothermic as you move right across a period because the effective nuclear charge increases, resulting in greater attraction for an electron.
To know more about Electron affinity, refer here:
https://brainly.com/question/977718#
#SPJ11
Write a balanced half-reaction for the oxidation of solid manganese dioxide to permanganate ion in acidic aqueous solution. be sure to add physical state symbols where appropriate.
Answers
The balanced oxidation half equation is given below:
MnO₂ + H₂O → MnO₄⁻ + H⁺ + 3 e⁻What are oxidation reactions?Oxidation reactions are reactions in which the oxidation of the species increases.
Oxidation reactions can involve addition of oxygen or electronegative elements to a substance or the removal of hydrogen or electropositive elements from a substance.
The balanced half-reaction for the oxidation of solid manganese dioxide to permanganate ion in acidic aqueous solution is given below:
MnO₂ + H₂O → MnO₄⁻ + H⁺ + 3 e⁻
In conclusion, the balanced oxidation half equation shows that three electrons were lost by the manganese (iv) ion to form manganese (vii) ion.
Learn more about oxidation half equation at: https://brainly.com/question/13186640
#SPJ1
In her lab, Dr. Yung has two samples that are both made up of hydrogen (H) and carbon (C) atoms. Some of the properties of each sample are listed below. A model of the group of atoms that repeats to make up sample 1 is shown below. Use the Modeling Tool: Two Samples at the Atomic Scale student sheet to create a model that represents a repeating group of atoms that could make up sample 2. Follow the instructions below.
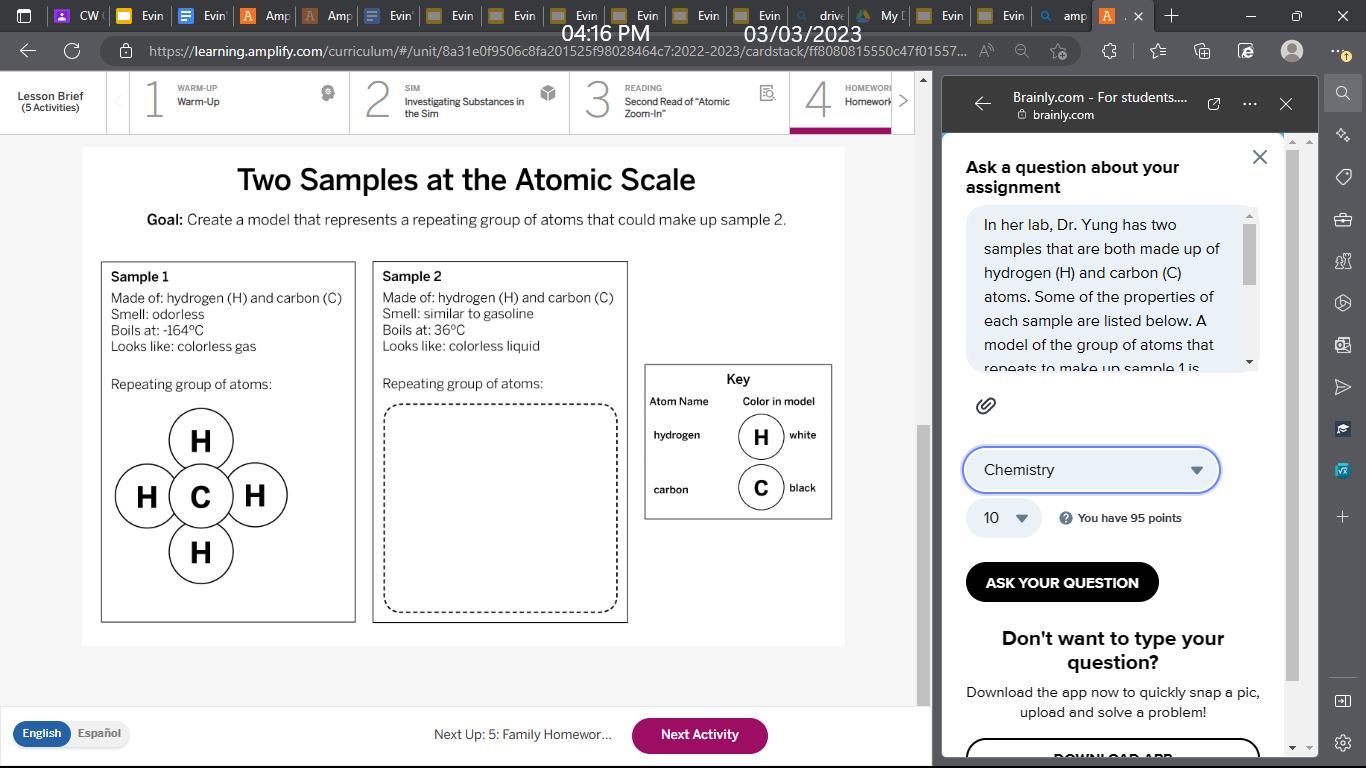
Answers
To create a model for a repeating group of atoms that could make up sample 2, is made up of hydrogen and carbon atoms, and it looks like a colorless liquid with a smell similar to gasoline. It has a low boiling point of 36°C.
what is hydrocarbon chain ?One possible repeating group of atoms for Sample 2 is a chain of carbon atoms bonded together with hydrogen atoms attached to the carbon atoms. This is called a hydrocarbon chain. The hydrocarbon chain can be represented as follows:
H H H H H H H H H H H H H H H H | | | | | | | | | | | | | | | | C-C-C-C-C-C-C-C-C-C-C-C-C-C-C-C
This repeating group of atoms is called a "linear alkane". The alkane has the chemical formula CnH2n+2, where n is the number of carbon atoms in the chain. For example, if there are 4 carbon atoms in the chain, the formula would be C4H10.
This model is consistent with the properties of Sample 2, as hydrocarbons are commonly used as fuels due to their flammability and low boiling points. The smell of hydrocarbons can vary, but some have a similar odor to gasoline. The low boiling point of hydrocarbons means they can exist as liquids at room temperature, which matches the description of Sample 2.
To know more about Hydrocarbon , visit :
https://brainly.com/question/16001047
#SPJ9
CH3COOH CH3COO– + H+
You start with 0.05 moles of acetic acid in 500 mL of water. At equilibrium, the pH of the solution is 2.873. What is the equilibrium constant of this reaction? Hint: You will need to calculate an antilog using a scientific calculator.
Answers
(a)
pH = 4.77
; (b)
[
H
3
O
+
]
=
1.00
×
10
-4
l
mol/dm
3
; (c)
[
A
-
]
=
0.16 mol⋅dm
-3
Explanation:
(a) pH of aspirin solution
Let's write the chemical equation as
m
m
m
m
m
m
m
m
l
HA
m
+
m
H
2
O
⇌
H
3
O
+
m
+
m
l
A
-
I/mol⋅dm
-3
:
m
m
0.05
m
m
m
m
m
m
m
m
l
0
m
m
m
m
m
l
l
0
C/mol⋅dm
-3
:
m
m
l
-
x
m
m
m
m
m
m
m
m
+
x
m
l
m
m
m
l
+
x
E/mol⋅dm
-3
:
m
0.05 -
l
x
m
m
m
m
m
m
m
l
x
m
m
x
m
m
m
x
K
a
=
[
H
3
O
+
]
[
A
-
]
[
HA
]
=
x
2
0.05 -
l
x
=
3.27
×
10
-4
Check for negligibility
0.05
3.27
×
10
-4
=
153
<
400
∴
x
is not less than 5 % of the initial concentration of
[
HA
]
.
We cannot ignore it in comparison with 0.05, so we must solve a quadratic.
Then
x
2
0.05
−
x
=
3.27
×
10
-4
x
2
=
3.27
×
10
-4
(
0.05
−
x
)
=
1.635
×
10
-5
−
3.27
×
10
-4
x
x
2
+
3.27
×
10
-4
x
−
1.635
×
10
-5
=
0
x
=
1.68
×
10
-5
[
H
3
O
+
]
=
x
l
mol/L
=
1.68
×
10
-5
l
mol/L
pH
=
-log
[
H
3
O
+
]
=
-log
(
1.68
×
10
-5
)
=
4.77
(b)
[
H
3
O
+
]
at pH 4
[
H
3
O
+
]
=
10
-pH
l
mol/L
=
1.00
×
10
-4
l
mol/L
(c) Concentration of
A
-
in the buffer
We can now use the Henderson-Hasselbalch equation to calculate the
[
A
-
]
.
pH
=
p
K
a
+
log
(
[
A
-
]
[
HA
]
)
4.00
=
−
log
(
3.27
×
10
-4
)
+
log
(
[
A
-
]
0.05
)
=
3.49
+
log
(
[
A
-
]
0.05
)
log
(
[
A
-
]
0.05
)
=
4.00 - 3.49
=
0.51
[
A
-
]
0.05
=
10
0.51
=
3.24
[
A
-
]
=
0.05
×
3.24
=
0.16
The concentration of
A
-
in the buffer is 0.16 mol/L.
hope this helps :)
The equilibrium constant of this reaction is 1.80×10-5
Given data,
pH of solution = 2.873
Number of moles of acetic acid (m) = 0.05 moles
Volume of water (V) = 500 mL = 0.5L
So, concentration (C) = m/V in lit = 0.05/0.5 = 0.1 M
Equilibrium constant ( K ) = \([CH_{3} COO-]\)×\([H+_{} ]\)/\([CH_{3} COOH]\)
Since, acetic acid is weak acid,
So, Equilibrium constant ( K ) = \([H+]^{2}\)/\([CH_{3} COOH]\) ....(i)
As the pH = 2.873, the \([H+_{} ]\) is antilog of -2.873 or 1.34×10-3 M.
Putting the value of concentration of \(H+_{}\) and \(acetic_{} acid\) in equation (i).
Equilibrium constant ( K ) = 1.80×10-5
What is weak acid ?The acid which is partially dissociates into ions on dissolving in aqueous solution is called weak acid.
Example: \(acetic_{} acid\).
To learn more about weak acid here.
https://brainly.com/question/12811944
#SPJ3
A compound composed of carbon and hydrogen with the formula CxHy was burned in a combustion reaction. The balanced equation is shown below.
2CxHy + 15O2 (g) → 12CO2 (g) + 6H2O (l)
Answers
A balanced chemical equation is a representation of a chemical reaction using chemical formulas and coefficients to show the identities and quantities of the reactants and products. The coefficients in a balanced chemical equation indicate the relative amounts of each substance involved in the reaction.
Chemical equation explained
The balanced chemical equation for the combustion of the compound with the formula CxHy is:
2CxHy + 15O2 (g) → 12CO2 (g) + 6H2O (l)
In this reaction, the compound reacts with oxygen gas to produce carbon dioxide and water. To balance the equation, the coefficients 2 and 15 are used for CxHy and O2, respectively, to ensure that the number of atoms of each element is the same on both the reactant and product sides of the equation.
The combustion of the compound results in the complete oxidation of the carbon and hydrogen atoms, producing carbon dioxide and water as the only products.
Therefore, specific values of x and y will depend on the identity of the compound, but then balanced equation above can be used to calculate the stoichiometry of the reaction and determine the amounts of reactants and products involved.
Learn more about chemical equation below.
https://brainly.com/question/11231920
#SPJ1
examples of fossil fuels (contain stored carbon)
Answers
Fossil fuels are formed over millions of years from the remains of dead plants and animals that have been buried under layers of rock and sediment.
These fuels contain stored carbon that was originally absorbed by the plants and animals during their lifetime. Examples of fossil fuels include coal, oil, and natural gas. When these fuels are burned for energy, the carbon is released into the atmosphere in the form of carbon dioxide, which contributes to climate change. Natural gas is a combustible mixture of hydrocarbons and other organic compounds that is found beneath the Earth's surface. Coal is a non-renewable fossil fuel that is used to generate electricity and heat, and is also used in the production of steel, cement, and other industrial products.
To learn more about carbon click here https://brainly.com/question/13719781
#SPJ11
A substance is highly malleable and has a shiny luster. Which of the following best explains the probable position of the substance in the periodic table? Group of answer choices Right or middle of the table because it is a non-metal. Left or middle of the table because it is a non-metal. Left or middle of the table because it is a metal. Right or middle of the table because it is a metal.
Answers
The position of a highly malleable and lustrous substance is the left or middle of the table because it is a metal.
These elements can be categorised into blocks in the periodic table based on the shell that an element's valence electron enters. Elements in the s block have valence electrons in the s orbital. The majority of these are metallic materials. Transition metals are more prevalent in the d block than in the p block.
What is malleability and lustrous ?In contrast to malleability, which allows metals to be beaten into sheets, and ductility, which allows metals to be made into wires, lustre is a quality that is associated to appearance, giving metals a shiny look.
Metals are lustrous (shiny), malleable (able to be hammered into thin sheets), ductile (able to be pulled into thin wires), and conduct heat and electricity. The majority of metals have a high melting point and are dense.Learn more about Malleability here:
https://brainly.com/question/23483808
#SPJ9
Answer:
Left or middle of the table because it is a metal.
Explanation:
A metal cube with a volume of 20 cubic centimeters has a mass of 157.48 gram. What is the density of the metal cube?
Answers
The density of the metal cube is 7.874 kg/m³
Density is a word we use to describe how much space an object or substance takes up means its volume in relation to the amount of matter in that object or substance means its mass and another way to put it is that density is the amount of mass per unit of volume and if an object is heavy and compact it has a high density
Here given data is metal cube with a volume = 20 cubic centimeters and mass = 157.48 gram so we have to find density of the metal cube = ?
So, density = mass/volume means ρ = m/V
Density = 157.48 gram/20 cubic centimeters
Density = 7.874 kg/m³
The density of the metal cube is 7.874 kg/m³
Know more about density
https://brainly.com/question/13695511
#SPJ1
What is the colour formed when N2O is bubbled into a solution of acidified FeSO4
Answers
Which has a free radical? ClO3- ; NO ; NO2- ; NO2 ?
Answers
NO and NO₂ are the free radicals.
The species which is having unpaired as well as odd number of electrons will be considered as a free radical. If the number comes out to be odd, the concerned species will be considered as a free radical. In case of NO₂, the total number of electrons is 23 (7 from nitrogen and 16 from two oxygen atoms) and in case of NO the total number of electrons is 15 (7 from nitrogen and 8 from oxygen). Hence, the species are free radical.
Whereas, in case of \(ClO3^{-}\), the total number of electrons is 42 (17 from chlorine and 24 from three oxygen and +1 for negative charge) and in case of \(NO2^{-}\)the total number of electrons is 24 (7 from nitrogen and 16 from oxygen and +1 for negative charge) There are even no. of electrons. Hence the \(ClO3^{-}\) and \(NO2^{-}\) are not a free radical.
To know more about free radicals here
https://brainly.com/question/29422803
#SPJ4
what are the properties of aluminium and their use
Answers
Answer: Answers are in bulleted lists.
Explanation: Aluminum...
has a low densityis non-toxichas a high thermal conductivityhas excellent corrosion resistancecan be easily cast, whether it's machined or formed.Uses:
Aeroplane PartsCansWindow framesBeer kegsFoils Kitchen utensilsHave a great day! :)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8
What element is represented by the ground state electron configuration shown above?
Answers
Answer:
Pd (palladium).
Explanation:
46 electrons total, this is representative of the Pd (palladium) element.
Two or more than two atoms with different physical or chemical properties can not combine together to form an element. Palladium (Pd) element is represented by the ground state electronic configuration.
What is element?Element generally consist of atoms or we can atoms combine to form element. Atoms of an element is always same, means all the properties of all atoms of one type of element is same.
The ground state electronic configuration of 1s² 2s² 2p⁶3s² 3p⁶4s² 3d¹⁰ 4p⁶ 5s² 4d⁸ shows total 46 electrons. The element with atomic number 46 is palladium, Pd. Palladium is a d block element. Palladium is found in group 8 and period four of periodic table.
Therefore, palladium (Pd) element is represented by the ground state electronic configuration.
To know more about element, here:
https://brainly.com/question/8460633
#SPJ3