Balance each equation by entering the correct coefficients.
NH3 +
O2 →
NO +
H2O
Answers
Balanced equation:
4 NH3 + 5 O2 = 4 NO + 6 H2O
Related Questions
How many moles of methane gas are in 44.8 L of CH at STP?
Answers
Answer:
2 mol CH 4
Explanation:
43.8 x L
---------------- = 2 mol CH 4
22.4 x L x mol - 1
How many atoms are there in the repeating peptide backbone units of proteins? Select one: a. 1 b. 2 c. 3 d. 4 e. 5
Answers
The repeating peptide backbone units of proteins contain 3 atoms. By understanding the structure of the "backbone" for peptides and proteins.
The structure of a peptide may be written very simply without illustrating the entire amide synthesis step.
The N-H 2, CH, C double bond O; N-H 2, CH, C double bond O; etc. repeating units make up the peptide backbone.
Nothing more than the use of the amide synthesis process results in the creation of peptides. The amide bond in peptides is often formed in the same sequence that the amino acids are listed. An amino acid's amine end (N terminal) is always on the left and its acid end (C terminal) is always on the right. The diagram on the left illustrates how to write the reaction between glycine and alanine to create the dipeptide glyclalanine. A water molecule is created when hydrogens (red) on the amine and oxygen (red) from the acid combine. The amide bond is created by the joining of the carboxyl oxygen (green) with the amine nitrogen (green).
Learn more about Peptide here:
https://brainly.com/question/30332053
#SPJ4
based on the balance reaction between iron and oxygen, how many electrons are transferred when 15 g of iron react?
Answers
12 electrons are transferred in this reaction when 15 g of iron react.
What is a chemical reaction?A chemical reaction is described as a process that leads to the chemical transformation of one set of chemical substances to another
The balanced chemical equation for the reaction between iron and oxygen is:
4 Fe + 3 O2 → 2 Fe2O3
The iron has a +3 oxidation state and the oxygen has a -2 oxidation state as products.
There are 4 iron atoms and 6 oxygen atoms in this reaction and if we multiply the oxidation state of each ion by the quantity of each gives us the number of electrons transferred.
Iron gives up (4)*(3) = 12 electrons and oxygen takes (6)*(2) = 12 electrons
Learn more about oxidation state at: https://brainly.com/question/25551544
#SPJ1
the half equivalence point is in the middle of the buffer region. in order to reach the half equivalence point in their titration, veronica needed to add 21.57 ml of koh to 50.00 ml of 0.467 m hf. what is the concentration of conjugate base at the half equivalence point?
Answers
The half equivalence point is the point in a titration where exactly half of the acid has reacted with the base, and the other half remains. At this point, the concentration of the acid and its conjugate base are equal.
The buffer region is part of the titration curve where the pH changes slowly with the addition of small amounts of acid or base. In order to calculate the concentration of the conjugate base at the half equivalence point, we need to first determine the number of moles of HF that Veronica started with. This can be calculated using the equation:
moles HF = Molarity x Volume (in liters)
moles HF = 0.467 mol/L x 0.0500 L
moles HF = 0.0234 moles
Since the half equivalence point is in the middle of the buffer region, Veronica must have added half of the amount of KOH required to reach the equivalence point. Therefore, we can calculate the amount of KOH added at the half equivalence point using the:
KOH added = 21.57 mL / 2
KOH added = 10.785 mL
We can convert this to volume in liters:
KOH added = 10.785 mL / 1000 mL/L
KOH added = 0.010785 L
We can now calculate the number of moles of KOH added at the half equivalence point using the equation:
moles KOH = Molarity x Volume (in liters)
moles KOH = 0.160 mol/L x 0.010785 L
moles KOH = 0.001727 moles
Since the reaction between HF and KOH is a 1:1 reaction, this means that 0.001727 moles of HF have reacted at the half equivalence point. This leaves 0.0234 - 0.001727 = 0.0217 moles of HF remaining.
Since the concentration of the conjugate base is equal to the concentration of the acid at the half equivalence point, we can use the equation:
Molarity = moles / Volume (in liters)
Molarity of conjugate base = 0.0217 moles / 0.0500 L
Molarity of conjugate base = 0.434 M
Therefore, the concentration of the conjugate base at the half equivalence point is 0.434 M.
To know more about half equivalence point
brainly.com/question/15867090
#SPJ11
What alkyl bromide should be used in the malonic ester synthesis of the following carboxylic acid? CH2CH2COH I. Br CH3Br IV. II. CH3CH2Br CH CH Br III. CH Br A) I B) 11 C) III D) IV E) V
Answers
The appropriate alkyl bromide to be used in the malonic ester synthesis of the given carboxylic acid, CH2CH2COH, is option III, CH3CH2Br.
The malonic ester synthesis is a method used to synthesize carboxylic acids by reacting an alkyl halide (in this case, an alkyl bromide) with diethyl malonate. The alkyl bromide serves as the alkylating agent in the reaction, introducing the desired alkyl group into the malonic ester.
In the given options, option III, CH3CH2Br, corresponds to ethyl bromide. This alkyl bromide can be used to alkylate the diethyl malonate, resulting in the desired carboxylic acid, CH2CH2COOH.
Option I, Br, is not a specific alkyl bromide and does not provide the necessary alkyl group for the synthesis.
Option II, CH CH Br, is a polybrominated compound and not suitable for the malonic ester synthesis.
Option IV is not provided in the question.
Option V is not provided in the question.
Therefore, the correct answer is option III, CH3CH2Br, which is the appropriate alkyl bromide to be used in the malonic ester synthesis of the given carboxylic acid.
Know more about Ester synthesis here:
https://brainly.com/question/31034180
#SPJ11
Which of the following is the smallest particle of matter?
A. Atom
B. Cell
C. Molecule
D. Organ
Answers
Answer:
A. Atom
Explanation:
Hope this helps !
A vessel with a volume of 26.9 L contains 2.80 g of nitrogen gas, 0.605 g of hydrogen gas, and 79.9 g of argon gas. At 25°C, what is the pressure in the vessel?
A)
75.5 atm
B)
0.183 atm
C)
2.55 atm
D)
2.18 atm
E)
58.7 atm
Answers
The pressure in the vessel is 2.18 atm. Answer choice (D) is correct.
To solve this problem, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to find the total number of moles of gas in the vessel:
n_total = n_N2 + n_H2 + n_Ar
We can find the number of moles of each gas using the given masses and their molar masses:
n_N2 = 2.80 g / 28.01 g/mol = 0.0999 mol
n_H2 = 0.605 g / 2.02 g/mol = 0.2995 mol
n_Ar = 79.9 g / 39.95 g/mol = 2.00 mol
n_total = 0.0999 mol + 0.2995 mol + 2.00 mol = 2.40 mol
Next, we can rearrange the ideal gas law to solve for the pressure:
P = nRT / V
We need to convert the temperature from Celsius to Kelvin:
T = 25°C + 273.15 = 298.15 K
We can substitute the values and solve for P:
P = (2.40 mol)(0.08206 L·atm/mol·K)(298.15 K) / 26.9 L = 2.18 atm
Therefore, the pressure in the vessel is 2.18 atm. Answer choice (D) is correct.
Learn more about pressure here:
https://brainly.com/question/15170210
#SPJ11
A surplus (having “extra”) or deficit (having “fewer”) of electrons?
Answers
Answer:
Charge
Explanation:
A surplus (having "extra") or deficit (having "fewer") of electrons possessed by an object. A charge can cause attractive or repulsive forces which can be observed in some cases (e.g. pith balls, bits of plastic).
which of the following is an alkaline earth metal?
A.Silicon (Si)
B.Aluminum (Al)
C.Carbon (C)
D.Magnesium (Mg)
Answers
Animation shows a complete revolution of the Earth around the sun. What happens during this time? The moon makes one complete phase change. The moon makes one complete phase change. Four seasons occur on Earth. Four seasons occur on Earth. Earth experiences a complete day. Earth experiences a complete day. Day turns to night.
Answers
Answer:
Four seasons occur on Earth
Explanation:
When the Earth has completely one revolution around the Sun, then it has completed 1 year, and four seasons have occurred in the meantime.
Four seasons occur on Earth when the Earth completes a revolution around the Sun.
The following points can be considered:
The Moon makes one complete phase change when the Moon revolves around the Earth. The Moon makes one complete phase change by a month. Four seasons occur on Earth as a result of the Earth completes one revolution around the Sun. The Earth experiences a complete day as a result of the Earth’s rotation along its own axis. The day turns to night as a result of the Earth’s rotation along its own axis.Therefore the correct answer is Four seasons occur on Earth.
Learn more about Earth:
https://brainly.com/question/18203196
How many moles are in 5.30 X 1023 molecules of H2O?
Answers
Answer:
0.880 (0.88039867109 to be exact)
Explanation:
To convert from molecules to moles, simply divide by Avogadro's number which is 6.02 x 10^23
So, 5.30x10^23/6.02x10^23 = 0.880
What gas must be present for burning to happen?
Answers
Answer:
oxygen should be present for burning to happen,
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in aqueous solutions. The definiton of pH is in terms of base 10 logarithms.

Answers
Answer:
a. pH = 2.22.
b. [H+] = 2.588 x 10⁻⁴ mol/L.
Explanation:
Acids and Bases => Calculating pH of Acids and Bases.
As we saw before, the formulas to find the pH based on the hydrogen ion concentration [H+], and to find the hydrogen ion concentration [H+] based on the pH are the following, respectively:
\(\begin{gathered} pH=-log\lbrack H^+], \\ \\ [H^+]=10^{-pH}. \end{gathered}\)So let's see each case:
a. To find the pH of an H+ concentration of 6.02 x 10⁻³ mol/L we use the pH formula:
\(\begin{gathered} pH=-log\lbrack6.02\cdot10^{-3}], \\ \\ pH=2.220\approx2.22. \end{gathered}\)The answer would be that the pH is 2.22.
b. To find the H+ concentration of a pH of 3.587, we use the [H+] formula:
\(\begin{gathered} \lbrack H{}^+]=10^{-3.587}, \\ \\ [H^+]=2.5882\cdot10^{-4}\text{ mol/L}\approx2.588\cdot10^{-4}\text{ mol/L.} \end{gathered}\)The answer would be that the hydrogen ion concentration [H+] = 2.588 x 10⁻⁴ mol/L.
what is the symbol for Lithium, Iron, and Helium
Answers
Lithium=li
Iron=Fe
Helium=He
I don't say u must have to mark my ans as brainliest but if u think it has really helped u plz don't forget to thank me....
Answer:
lithium symbol=li helium= he and iron =i
Arabitol is a sugar alcohol derived from arabinose by catalytic hydrogenation. Is it chiral or meso? chiral он ОН НО ОН Erythritol Erythritol is a sugar alcohol derived from erythrose by catalytic hydrogenation. Is it chiral or mesey chiral Submit Answer Try Another Version 1 item attempt remaining 44°F -- A
Answers
Both Arabitol and Erythritol are chiral due to the presence of multiple chiral centers in their structures.
Arabitol is derived from arabinose through catalytic hydrogenation. It is chiral because it contains multiple chiral centers, leading to stereoisomers that are non-superimposable mirror images of each other.
Erythritol is derived from erythrose by catalytic hydrogenation. It is also chiral because it has multiple chiral centers, resulting in different Arabitol stereoisomers that cannot be superimposed onto each other.
For more information on Chiral nature of Arabitol and Erythritol refer https://brainly.com/question/9522537
#SPJ11
How many grams of kmno4 correspond to 3.13 x 1022 formula units of kmno4? avogadro's number is 6.022 x 1023.
Answers
8.21g of KMnO₄ correspond to 3.13 x 10²² formula units of KMnO₄? avogadro' s number is 6.022 x 10²³
Here,3.13 x 10²² formula units of KMnO₄=1 mole of KMnO₄/avagadro no
1 mole of KMnO₄/6.022 x 10²³
= 3.13 x 10²²/6.022 x 10²³
= 5.197moles of KMnO₄
Now KMnO₄ has molar mass is 158.034g/mol
5.197moles of KMnO₄=158.034/1 mole of KMnO₄
158.034×5.197=821.1g of KMnO₄
Know more about grams of KMnO₄
https://brainly.com/question/28494037
#SPJ4
what best describes how an ionic bond forms
Answers
The metal atom forms positive ions called cations and the non-metal atoms form negative ions known as anions.
Both types of ions are stable with filled outer-shell electrons!
water can dissolve ionic compounds because a) water is a molecular compound. b) water is made up of hydrogen and oxygen. c) water is an acid. d) water is a polar molecule.
Answers
d. water is a polar molecule that's why it can dissolve ionic compounds.
The polarity of water refers to its molecular structure, where the oxygen atom has a partial negative charge and the hydrogen atoms have partial positive charges. This makes water a polar molecule, meaning that it has a positive and negative end. Due to its polarity, water is able to dissolve ionic and polar substances, making it an excellent solvent. This property of water is important in many biological and chemical processes.
Learn more about polar substance here:
https://brainly.com/question/11405437
#SPJ4
Can somebody Please Help
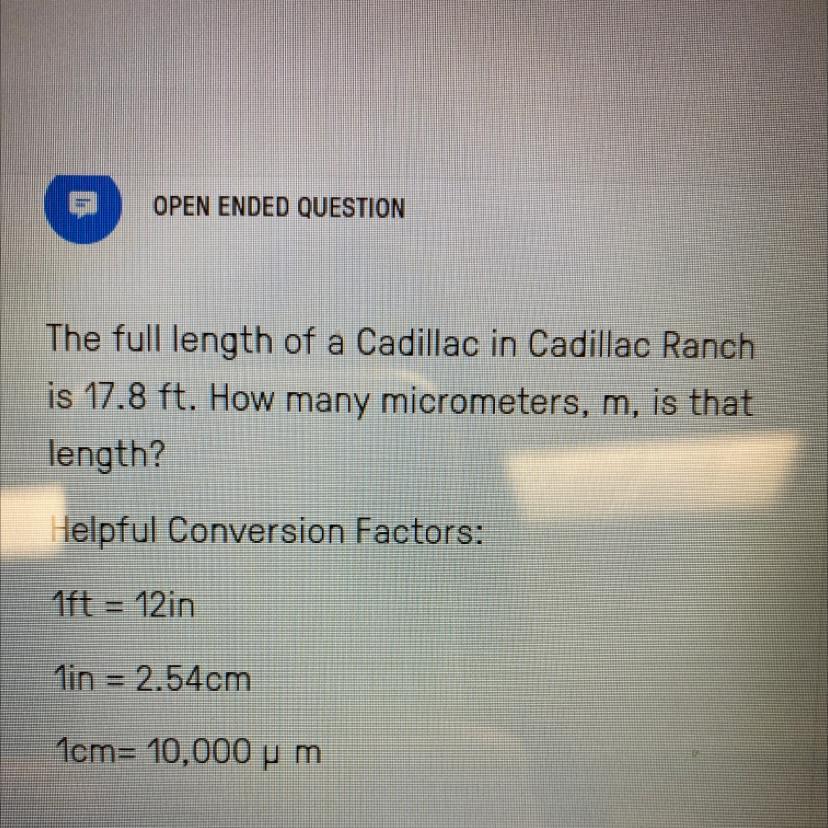
Answers
Answer:
5425440 μm
Explanation:
From the question given above,
Length (in ft) = 17.8 ft
Length (in μm) =?
Next, we shall convert 17.8 ft to in. This can be obtained as follow:
1 ft = 12 in
Therefore,
17.8 ft = 17.8 ft × 12 in / 1 ft
17.8 ft = 213.6 in
Next, we shall convert 213.6 in to cm. This can be obtained as follow:
1 in = 2.54 cm
Therefore,
213.6 in = 213.6 in × 2.54 / 1 in
213.6 in = 542.544 cm
Finally, we shall convert 542.544 cm to μm. This can be obtained as follow:
1 cm = 10⁴ μm
Therefore,
542.544 cm = 542.544 cm × 10⁴ μm / 1 cm
542.544 cm = 5425440 μm
Therefore, 17.8 ft is equivalent is equivalent to 5425440 μm
VALENCE STRUCTURE the example in the lesson, 0.10 mole of sodium chloride or magnesium chloride or aluminum chloride was added to one liter of water. How many moles of each chloride are in one milliliter of the respective solutions
Answers
In one milliliter of each solution, there are 0.1 millimoles of the respective chloride.
In one liter of water, 0.10 mole of either sodium chloride, magnesium chloride or aluminum chloride was added. Therefore, to determine the number of moles of each chloride present in one milliliter of the solutions, we will have to calculate the molarity of the solutions using the formula;
Molarity = moles of solute/liters of solution.
Once we have obtained the molarity of the solutions, we can convert to moles per milliliter by multiplying by 0.001.
Let's calculate the molarity of each solution:
Molarity of NaCl solution = 0.10 moles/1 liter = 0.10 M
Molarity of MgCl2 solution = 0.10 moles/1 liter = 0.10 M
Molarity of AlCl3 solution = 0.10 moles/1 liter = 0.10 M
To convert to moles per milliliter, we multiply each molarity by 0.001.
Moles of NaCl in 1 mL of solution = 0.10 M x 0.001 L = 0.0001 moles or 0.1 mmol
Moles of MgCl2 in 1 mL of solution = 0.10 M x 0.001 L = 0.0001 moles or 0.1 mmol
Moles of AlCl3 in 1 mL of solution = 0.10 M x 0.001 L = 0.0001 moles or 0.1 mmol
Therefore, in one milliliter of each solution, there are 0.1 millimoles of the respective chloride.
In conclusion, we can say that the number of moles of each chloride present in one milliliter of the respective solutions is 0.1 millimoles. This was calculated by first finding the molarity of each solution using the formula Molarity = moles of solute/liters of solution. The molarity of each solution was found to be 0.10 M. We then converted the molarity of each solution to moles per milliliter by multiplying by 0.001 L. This gave us the number of moles of each chloride present in one milliliter of the respective solutions.
Learn more about molarity visit:
brainly.com/question/31545539
#SPJ11
Question 4(Multiple Choice Worth 2 points)
(01.05 LC)
What property of matter takes up space?
O Mass
O Temperature
O Volume
O Weight
Answers
Answer:
Its not mass
Explanation:
X Mass out.
is the ability of a substance to reflect light a chemical property
Answers
Answer:
If it reflects all light, it looks white. Color can help identify a material and, while it is a physical property, it can be used together with chemical experiments when the experiments produce a known material with a specific color.
Explanation:
The ability to reflect light is the inherent and fundamental property of that substance.
What type of object reflects light?When waves of light (and other electromagnetic radiation) come into contact with a surface or other boundary that does not absorb the radiation's energy, the waves bounce off the surface and back into space. The light wave that is coming in is known as the incident wave, and the wave that is being reflected off of the surface is known as the reflected wave. As seen for the operation of a flashlight beam on a smooth, flat mirror, visible white light that is focused onto the surface of a mirror at an angle (incident) is reflected back into space by the mirror surface at another angle (reflected) that is equal to the incident angle.For visible light and all other wavelengths of the electromagnetic radiation spectrum, the angle of incidence is the same as the angle of reflection. This idea is frequently referred to as the Law of Reflection. It is significant to notice that because the light is not "bent" or refracted and is being reflected at all wavelengths equally, it is not broken up into its individual hues. Practically all surfaces will reflect light to some extent, particularly smooth surfaces, like a glass mirror or polished metal, work best for doing so.To learn more about reflective objects, refer to
https://brainly.com/question/3200266
#SPJ2
What is an experimental error
Answers
Answer:
Explanation: errors in judgment of an observer when reading the scale of a measuring device to the smallest division. 2. Environmental. For example, unpredictable fluctuations in line voltage, temperature, or mechanical vibrations of equipment.
Which atoms are indicated by the following configuration?
A.[He]2s^1.
B.[Ne]3s^2 3p^5.
C.[Ar]4s^2.
Answers
\(\\ \sf\longmapsto [He]2s^1=Lithium \)
\(\\ \sf\longmapsto [Ne]3s^23p^5=Chlorine\)
\(\\ \sf\longmapsto [Ar]4s^2=Calcium\)
how much volume does a 3.2 M solution of NaCl occupy with 50 moles of NaCl in solution?
Answers
Answer:
data given
molarity 3.2m
moles 50mol
Required volume
Explanation:
from
molarity =mole/volume
3.2=50/v
v=15.62
:.volume is15.62dm^3
Compare and contrast conventional and convectional oven
Answers
Answer:
The conventional oven and the convection oven are confused with each other due to their similar look and style, and a lot of their functioning is quite the same as well.
The conventional ovens have existed for over half a century, and have derived their functioning from traditional ovens that are over 4,000 years. They function with a bottom-up heat transfer from a fixed burner source.
The convection ovens were made recently as a way to get better heating results for all types of dishes and recipes, with the introduction of a fan system along with an exhaust which allows the circulation of hot air around the cavity of the oven.
Explanation:
What are examples of carbon emissions?.
Answers
Scope 1 emissions include things like gas stoves and personal vehicles. Scope 2 emissions include things like businesses that produce carbon emissions but also buy power.
Exhaust gas, also referred to as vehicle emissions, can contain a variety of pollutants and greenhouse gases. Flue gases, which are released through the plant's smokestack, are also produced by power plants that burn fossil fuels, such as coal-fired power plants or natural gas power plants. They are similar to exhaust gases in composition. Having the chemical symbol C and atomic number 6, carbon is an element with the meaning "coal" in Latin. It is nonmetallic and tetravalent, meaning that four of its atom's electrons can be used to create covalent chemical connections. Is called as carbon.
Learn more about carbon here
https://brainly.com/question/22530423
#SPJ4
When producing XY as shown in the equation, the energy of the reactants is 732 kJ/mol and the total bond energy of the product side is 1256 kJ/mol.
2X + Y2 --> 2XY
What is the total energy of the reaction?
show your work with picture provided

Answers
The reaction has an 836 kJ/mol total energy.
What does H stand for?The reaction is endothermic if the change in enthalpy is positive. A lower enthalpy indicates a process using less energy. Enthalpy (H) is a measure of the overall heat content of the system.
The difference between the energies of the reactants and products can be used to compute the reaction's overall energy.
energy required to break the bonds in Y2 = (732 kJ/mol) × 2 = 1464 kJ/mol
Determine the energy produced during the products' bond formation.
energy released by forming the bonds in XY = (1256 kJ/mol) / 2 = 628 kJ/mol
Determine the reaction's overall energy.
Total reaction energy equals energy released by creating the bonds in XY minus energy expended to break the bonds in Y2.
total energy of the reaction = 1464 kJ/mol - 628 kJ/mol
total energy of the reaction = 836 kJ/mol
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
Which orbital is portrayed on the right?

Answers
A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.
What's the appearance of the p orbital?A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.Each shell can only carry a certain amount of electrons: the first shell can hold two electrons, the second shell can hold eight electrons (2 + 6) and so on, the third shell can hold 18 electrons (2 + 6 + 10).To learn more about orbital refer to:
https://brainly.com/question/20319149
#SPJ1
2. Solid sodium reacts with gaseous chlorine to produce sodium chloride.