Atmosphere
B
Ocean
Land
с
Which arrow or arrows represents a change of state from a liquid to a gas caused by the
sun's energy?
A. arrow A
B. arrow B
C. arrow A and arrow B
D. arrow B and arrow C
Answers
Related Questions
what word describe how fast or how slow a reaction is
Answers
Answer:
WHAT SORT OF WAYS CAN WE MEASURE THE SPEED OF A CHEMICAL REACTION? The phrase 'rate of reaction' means 'how fast or how slow is the reaction' or 'the speed of the reaction'. It can be measured as the 'rate of formation of product' (e.g. collecting a gaseous product in a syringe) or the 'rate of removal of reactant'.
hope it helps!
please mark as the brainliest!
Calculate the molar mass of CC14
Answers
The molar mass of CCl₄ is 154 g/mol
Molar mass is the mass in grams of one mole of a substance and is given by the unit g/mol.
It is calculated by taking the sum of atomic masses of all the elements present in the given formula.
A mole is defined as the amount of substance containing the same number of atoms, molecules, ions, etc. as the number of atoms in a sample of pure 12C weighing exactly 12 g.
Atomic mass of C = 12
Atomic mass of Cl = 35.5
Molar mass = 12 + (35.5 × 4)
= 154 g/mol
Learn more about Molar mass, here:
https://brainly.com/question/12127540
#SPJ1
hello, do you think you know the order of this?

Answers
Answer:
1. A
2. B
3. D
4. C
Explanation:
Just trust me. Good luck on your test! (I go to K12 as well.)
What is the frequency of a photon with an energy of 4.56 × 10^-19 J?OA. 6.88 x 10^14 HzOB. 6.42 x 10^14 HzOC. 4.36 x 10^14 HzOD. 5.10 x 10^14 Hz
Answers
So,
There's an equation that we could use in order to find frequency, and it is the next one:
This equation tells us that the energy of the photon is equal to the product of the Plank constant (h), which is 6.626*10^-34 J.s, and the frequency.
In this problem, we know the value of E and the value of h, so we need to solve for v:
Therefore, the correct answer option is A.


4) How many atoms are present in a sample of oxygen, O2 with 12.222 moles?
Answers
Answer:
two oxygen atoms
13. Argentaffin granules present in cells of the gastrointestinal tract are best preserved with:
a. absolute alcohol
b. Carnoy solution
c. 10% NBF
d. formalin-alcohol
Answers
Argentaffin granules are darkly staining granules present in cells of the gastrointestinal tract that contain the neurotransmitter serotonin. These granules play a crucial role in the regulation of gastrointestinal functions such as peristalsis and secretion.
To preserve argentaffin granules in cells of the gastrointestinal tract, the best method is to use the Carnoy solution. Carnoy solution is a fixative that contains a mixture of ethanol, chloroform, and acetic acid.
It is a rapid and efficient fixative that provides excellent preservation of cellular morphology and tissue architecture. Carnoy solution has been shown to effectively fix and preserve argentaffin granules in cells of the gastrointestinal tract.
Absolute alcohol and formalin-alcohol are commonly used fixatives but are not ideal for preserving argentaffin granules. 10% NBF (neutral buffered formalin) is a commonly used fixative in histology and is effective in preserving tissue morphology, but it is not the best option for preserving argentaffin granules.
In conclusion, to best preserve argentaffin granules present in cells of the gastrointestinal tract, the Carnoy solution is the ideal fixative to use.
Learn more about argentaffin granules here:
https://brainly.com/question/30693171
#SPJ11
What is the molality of a solution consisting of 44.0 mL of benzene (C₆H₆; d = 0.877 g/mL) in 167 mL of hexane (C₆H₁₄;d = 0.660 g/mL)?
Answers
Molarityof the solution will be 907.35 m.
What is Molality?Molaity is the no. Of moles of an compound is present in 1 kg solution .
Molality= no.0f moles / mass of the solution (in kg)
The SI unit of molality is m .
We have given here ,
Volume of benzene= 44.0ml
Density of benzene =0.877g/ mol
Volume of hexane =167 ml
Density of hexane =0.660g/ mol
Mass of benzene = 44×0.877
. →38.588 g
Mass of hexane→167×660
. → 110.220g
mass of the solution→148.808g
molar mass of the solution→78+ 86
. →164g/ mol
Molality = 148.808/ 164×1000
→907.35m
So , molality will be 907.35 m
to learn more about Molality click here https://brainly.com/question/26921570
#SPJ4
how do you balance _KCIO3 + __P4 → _P4010 + ___KCI
Answers
Under the topic of Investments in Equity and Debt Security, describe 3 pros and cons of investing in property.
Answers
Investing in property can offer several advantages and disadvantages. Here are three pros and three cons of investing in property:
Pros of Investing in Property:
Potential for Appreciation: One of the main advantages of property investment is the potential for property value appreciation over time. Real estate properties, especially in desirable locations, have the potential to increase in value, allowing investors to build wealth and generate a return on their investment.
Rental Income: Property investment can provide a steady stream of rental income. By purchasing residential or commercial properties and renting them out to tenants, investors can generate regular cash flow, which can be particularly beneficial for long-term financial planning or as an additional income source.
Portfolio Diversification: Investing in property allows for diversification of an investment portfolio. Real estate is considered an alternative asset class that can help balance the risk and return profile of an investment portfolio that may primarily consist of stocks and bonds. Adding property investments can reduce exposure to volatility in other markets.
Cons of Investing in Property:
High Initial Costs: Acquiring property often requires a substantial upfront investment. Investors need to consider the down payment, closing costs, property maintenance expenses, and potential renovation costs. This can be a barrier for individuals with limited capital or seeking more liquid investments.
Market Volatility and Liquidity: The property market can be subject to fluctuations and cycles, which can affect property values. Unlike stocks or bonds, buying or selling property may take time and effort, reducing liquidity. In certain market conditions, it may be challenging to sell a property quickly if needed.
Management and Maintenance: Property investment requires active management and ongoing maintenance. Landlords are responsible for property upkeep, repairs, and addressing tenant issues. Managing rental properties can be time-consuming and may involve dealing with various challenges such as vacancies, property damage, and rental disputes.
Learn more about Investments, here:
https://brainly.com/question/15105766
#SPJ4
What is the energy of a photon that emits a light of frequency 6.42 x 1014 Hz?

Answers
Answer:
Option B. 4.25×10¯¹⁹ J
Explanation:
From the question given above, the following data were obtained:
Frequency (f) = 6.42×10¹⁴ Hz
Energy (E) =?
Energy and frequency are related by the following equation:
Energy (E) = Planck's constant (h) × frequency (f)
E = hf
With the above formula, we can obtain the energy of the photon as follow:
Frequency (f) = 6.42×10¹⁴ Hz
Planck's constant (h) = 6.63×10¯³⁴ Js
Energy (E) =?
E = hf
E = 6.63×10¯³⁴ × 6.42×10¹⁴
E = 4.25×10¯¹⁹ J
Thus, the energy of the photon is 4.25×10¯¹⁹ J
Answer:
B. 4.25 x 10-19J is correct via a p e x
Explanation:
Calculate the molarity (moles/L) of acetic acid in vinegar: Use the molar mass of acetic acid to convert your molarity value above to grams of acetic acid per mL Take this number times [00 to get & percent acetic acid in vinegar: (The result should be close to 5%.)
Answers
Calculating the molarity of acetic acid in vinegar:
Molarity (M) = (number of moles of solute) / (volume of solution in liters)
What is molar mass?The molar mass is the same as mass number if it is only one element with no subscripts.
the mass of acetic acid in the vinegar will be determined first:
Mass = volume (L) × density (g/mL)
Mass = 1 L × 1.05 g/mL
Mass = 1.05 g/L
Then, the moles of acetic acid can be calculated using the molar mass of acetic acid:
Moles = mass (g) / molar mass
Moles = 1.05 g / 60.05 g/mol
Moles = 0.01748 mol
Acetic acid molarity = 0.01748 mol / 1 L
= 0.01748 M
Calculating the percentage of acetic acid in vinegar:
% acetic acid = (mass of acetic acid/volume of vinegar) × 100%
= (1.05 g / 100 mL) × 100%
= 1.05%
Therefore, the result of the calculation will be close to 1.05%, not 5%.
To know more about molarity:
https://brainly.com/question/19517011
#SPJ11
This diagram illustrates the life cycle of a tomato plant. Which stage in this plant life cycle is the adult? Tomato Plant Life Cycle
Answers
Answer:
the answer is A
Explanation:
hope it hlp
Match each renewable energy source with the correct description. solar cell converts sunlight into electricity geothermal energy turns turbines along coastlines wave water falling through dams turns turbines, generates electricity hydroelectricity o heat produced within the Earth
Answers
Answer:
Explanation:
solar cell - converts sunlight into electricity
geothermal energy - heat produced within the Earth
wave energy - turns turbines along coastlines
hydroelectricity - water falling through dams turns turbines, generates electricity
Answer:
Explanation:
The renewable energy sources are: solar cell, geothermal, wave and hydroelectricity. Their descriptions are as follows:
converts sunlight into electricity -> solar cell
energy turns turbines along coastlines -> wave
water falling through dams turns turbines, generates electricity -> hydroelectricity
heat produced within the Earth -> geothermal
NAME: Madsen Binette
CLASS: Blue 4
Percent yield practice
1. Consider the following balanced equation
2 Al + 6 HBr - →2 AlBr3 + 3 H₂
A) If 86.88 grams of Aluminum (Al) reacts with 401.31 grams of
Hydrogen Bromide (HBr) how many grams of H₂ will be produced?
B) which is the limiting reactant?
C) what is the percent yield
Answers
Answer:
I dont understand nd also i need to message u privately
Identify the limiting reactant when 9.0 L CS reacts with 18.0 L O .CS2(g) + 3O2(g) CO2(g) + 2SO2(g)
Answers
The limiting reactant in the given reaction is CS (carbon disulfide).
To determine the limiting reactant, we need to compare the amount of each reactant used with the stoichiometry of the balanced equation. Since the balanced equation shows that the molar ratio between CS and O2 is 1:3, we need to convert the given volumes to moles using the ideal gas law. After comparing the moles of CS and O2, we find that CS is the limiting reactant.
Therefore, CS is the limiting reactant in the reaction. It means that all the CS will be consumed before the O2 is completely utilized, limiting the amount of product that can be formed.
To know more about reactant click here:
https://brainly.com/question/30129541
#SPJ11
Identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent.
SO2+ 2H2S----> 3S + 2H2O
Answers
Explanation:
here is the answer bae. Feel free to ask for more chem help

is this reaction oxidation or reduction
CH2=CH2 + OsO4 yields to HOCH2CH2OH
Answers
Answer:
it's definitely a oxidation reaction because here the oxygen contained OH group present in yeild
What must happen to uranium before it can be used as a fuel source?
A. No changes are necessary.
B. Stable U-235 must be enriched with 3% radioactive U-238.
C. Stable U-238 must be enriched with 3% radioactive U-235.
D. Unstable U-235 must be enriched with 5% U-238 to stabilize it.
Answers
Answer:
D
Explanation:
Before it can be used in a reactor for electricity generation, however, it must undergo a series of processes to produce a useable fuel. For most of the world's reactors, the next step in making the fuel is to convert the uranium oxide into a gas, uranium hexafluoride (UF6), which enables it to be enriched.
Unstable U-235 must be enriched with 5% U-238 to stabilize it. Hence, option D is correct.
What is radioactive?Radioactivity is the phenomenon of the spontaneous disintegration of unstable atomic nuclei into atomic nuclei to form more energetically stable atomic nuclei.
Before uranium can be used in a reactor for electricity generation, however, it must undergo a series of processes to produce a useable fuel.
For most of the world's reactors, the next step in making the fuel is to convert the uranium oxide into a gas, uranium hexafluoride (UF6), which enables it to be enriched.
Hence, option D is correct.
Learn more about radioactive here:
https://brainly.com/question/1770619
#SPJ2
in which solution is the [h3o+] less than 0.250 m?
Answers
The [H3O+] concentration refers to the concentration of hydronium ions in a solution.
To find the solution in which the [H3O+] is less than 0.250 m, you need to calculate the concentration of hydronium ions in each solution and compare it to 0.250 m.
The solution with a concentration of [H3O+] less than 0.250 m is the one where the hydronium ion concentration is lower than that value.
To determine the solution in which the [H3O+] is less than 0.250 m, you need to compare the [H3O+] concentration of each solution to 0.250 m. The solution with a [H3O+] concentration lower than 0.250 m will be the one in which the hydronium ion concentration is less than that value. It is important to note that the pH of a solution is directly related to the concentration of hydronium ions, so a lower pH corresponds to a higher [H3O+] concentration and vice versa.
The solution with a [H3O+] concentration less than 0.250 m is the one in which the hydronium ion concentration is lower than that value. To determine the [H3O+] concentration, you can use the pH value, which is inversely related to the [H3O+] concentration. Therefore, a lower pH indicates a higher [H3O+] concentration and vice versa.
To know more about hydronium ions visit:
brainly.com/question/14619642
#SPJ11
you find a piece of cloth painted with organic dye. by analyzing the dye, you find that only 71 % of the carbon-14 originally in the dye remains. when was the cloth painted? express your answer in years to two significant figures.
Answers
The cloth was painted with organic dye approximately 2,300 years ago ( rounded to two significant figures ).
To determine when the cloth was painted with organic dye, we can use the half-life of carbon-14 and the given information that 71% of the carbon-14 remains. The half-life of carbon-14 is 5,730 years.
Step 1: Calculate the decay factor.
Decay factor = remaining percentage / 100
Decay factor = 71% / 100 = 0.71
Step 2: Use the decay formula.
N(t) = N0 * (1/2)^(t / half-life)
0.71 = (1/2)^(t / 5730)
Step 3: Solve for t (time in years).
t / 5730 = log(0.71) / log(1/2)
t = 5730 * (log(0.71) / log(1/2))
t ≈ 2349.27 years
Expressing the answer to two significant figures, the cloth was painted approximately 2,300 years ago.
Learn more about organic dye at https://brainly.in/question/23188527
#SPJ11
What is the electrochemistry underlying the electrical current in a neuron as it is stimulated? Potassium channels closing to keep potassium inside the cell Potassium channels opening to allow potassium to enter the cell Sodium channels opening to allow sodium to exit the cell Sodium channels opening to allow sodium to enter the cell Question 2 The MOST FUNDAMENTAL variable that can probably explain the evolution of bigger brains is related to whether the animal is a dietary generalist or specialist the overall ecological complexity that the animal deals with whether the animal is solitary or social whether the animal is monogamous or polygynous
Answers
The electrochemistry underlying the electrical current in a neuron as it is stimulated involves sodium channels opening to allow sodium to enter the cell.
2. The most fundamental variable that can likely explain the evolution of bigger brains is the overall ecological complexity that the animal deals with.
During neuron stimulation, an action potential is generated. This process involves the depolarization of the neuron's membrane, which is achieved by the influx of positively charged ions, primarily sodium ions (Na+). When a neuron is stimulated, voltage-gated sodium channels in the cell membrane open, allowing sodium ions to rapidly enter the cell. This influx of positive charge depolarizes the membrane, creating an electrical current that propagates along the neuron.
Regarding the second question, the most fundamental variable that can likely explain the evolution of bigger brains is the overall ecological complexity that the animal deals with. Animals that inhabit complex and challenging environments often require enhanced cognitive abilities to navigate and respond to their surroundings effectively. The ecological complexity, such as varied food sources, social interactions, and environmental stimuli, can drive the evolutionary pressure for larger brain size and increased cognitive capacity. While factors like diet, sociality, and mating strategies may play a role, the overall ecological complexity is considered a crucial determinant of brain evolution.
To know more about electrochemistry, click here, https://brainly.com/question/32766737
#SPJ11
define a shadow plz help
Answers
Answer:
A shadow is that thing that follows you everywhere when the sun is out. It's creepy!
Explanation: Hope I helped!
Answer:
A shadow is a dark form that you cannot touch, hear, feel, or taste. It's simply formed when an object blocks a source of light.
which of the following is true for a chemical reaction at equilibrium? group of answer choices the rates of the forward and reverse reactions are equal. the concentrations of products and reactants are still changing. all reaction has ceased. the reaction has gone to completion to products. the amount of reactant(s) remaining is always equal to the amount of product(s) formed.
Answers
The right response is that the forward and reverse reaction rates are equivalent.
The concentrations of reactants and products are no longer changing macroscopically when the rate of the forward reaction equals the rate of the reverse reaction. On a microscopic level, there might still be very slight variations.
As some reactant(s) will remain at equilibrium, the reaction has not completely ended.
In a chemical reaction, the forward reaction rate is the rate at which reactants are converted into products, while the reverse reaction rate is the rate at which products are converted back into reactants.
The reverse reaction rate can be influenced by factors such as temperature, pressure, and the concentrations.
To know more about equilibrium here
https://brainly.com/question/517289
#SPJ4
4) Calculate the net force *
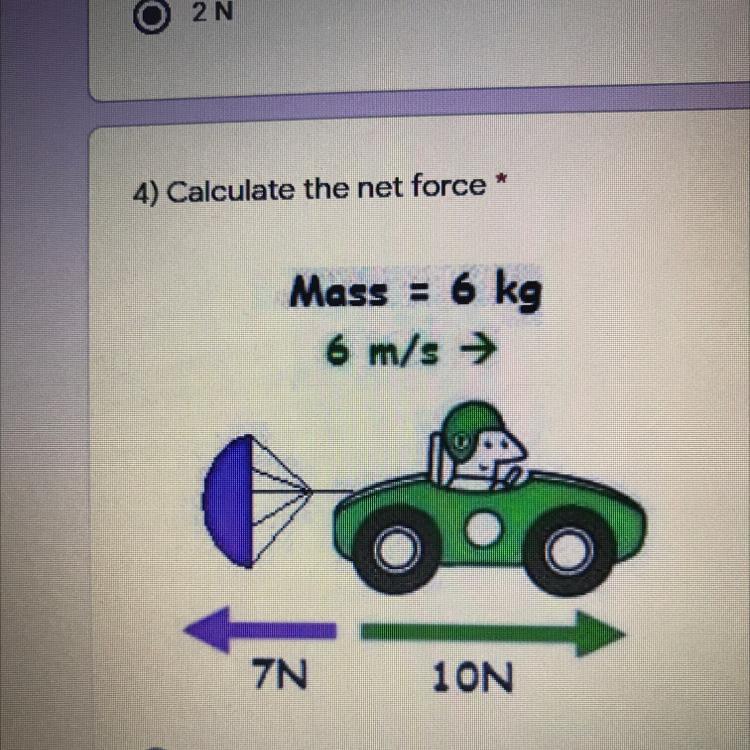
Answers
3 N
10-7= 3
the arrows are opposing each other.
Answer:
The net or resultant force depende on the direction we can easily understood the directions are opposite .Force is vector so if vectors are opposite we difference the two vectors.
10N-7N=3N
Someone pls help me I will make you brain

Answers
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
Which equilibrium constant represents a reaction that favors the reactants to the greatest extent?
Answers
Answer: If the value of K is less than 1, the reactants are favored
Explanation:
How many milliliters of 0.25M H2SO4 can be prepared from 57 mL of a 3.0M solution of H2SO4?
Answers
Answer:
Why ? Because 1 molecule of H2SO4 gives 2 H+ ions per molecule while only one H+ ion is required to neutralize 1 molecule of KOH. So, 1 molecule of H2SO4 can neutralize 2 molecules of KOH. Hence, we would require 525 ml of 0.03 M H2SO4 to neutralize 525 ml of 0.06 M KOH. How will we prepare 525 ml of 0.03 M H2SO4 ?
Explanation:
Now, we have 0.025 M H2SO4 and we do not know how much volume we have.
We will use the standard N1 X V1 = N2 X V2 for this calculation.
N1=0.025 M; V1=unknown; N2=0.03 M and V2=525 ml.
So V1= (0.03 X 525)/(0.025) = 630 ml.
According to the molar concentration, 684 ml of 0.25 M H₂SO₄ can be prepared from 57 mL of a 3.0 M solution of H₂SO₄.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.In case of 2 solutions,it is calculated as, M₁V₁=M₂V₂ substitution gives V₁=3×57/0.25=684 ml.
Thus, 684 ml of 0.25 M H₂SO₄ can be prepared from 57 mL of a 3.0 M solution of H₂SO₄.
Learn more about molar concentration,here:
https://brainly.com/question/15532279
#SPJ3
What is the mass of sodium hydroxide in a solution that has a total mass of 5,500g and is 22% NaOH by mass
Answers
Solution = 5,500g
NaOH = 22% of 5,500g
Therefore mass of NaOH is :
0.22 × 5,500g
1,210g
Meaning 1,210 grams inside the solution is Sodium hydroxide
The mass of sodium hydroxide in a solution that has a total mass of 5,500g and is 22% NaOH by mass is 121,000 g.
How do we calculate mass percent?Mass percent of any substance present in any solution will be calculated as:
Mass % = (Mass of substance) / (Mass of solution)
In the question given that,
Mass percent of NaOH = 22%
Mass of solution = 5,500 g
On putting these values on the above equation we get,
Mass of NaOH = (22)×(5,500) = 121,000 g
Hence required mass of NaOH is 121,000 g.
To know more about mass percent, visit the below link:
https://brainly.com/question/10031774
a building supply company has many high volume customers and many low-volume customers. a crm system
Answers
A CRM system can help a building supply company manage their many high volume and low-volume customers more effectively.
By tracking customer interactions and purchases, a CRM system can help identify trends and opportunities for upselling or cross-selling to both types of customers.
Additionally, a CRM system can help a company provide more personalized and efficient customer service by allowing representatives to quickly access customer information and preferences.
This can lead to increased customer loyalty and satisfaction, as well as improved sales and profitability for the company.
To know more about CRM system visit:
https://brainly.com/question/30539455
#SPJ11