Answers
Answer:
21 is the max but with the bread ypu could make 22 if you had 3 more cheese
Related Questions
Which statement is true about a neutral solution? (5 points)
Its pH is less than 7.
Its pH is greater than 7.
It has the same concentration of hydronium and hydroxide ions.
It has a greater concentration of hydroxide ion than hydronium ions.
Answers
The statement "It has the same concentration of hydronium and hydroxide ions" is true about a neutral solution.
What is a neutral solution?If you've ever wondered what constitutes a neutral solution - it's one with an ideal pH balance set at precisely 7. Referred to as a 'neutral' because it doesn't lean towards acidity or alkalinity due to an equal concentration rate between hydronium ions (H+) and hydroxide ions (OH-).
Noteworthy examples can include distilled water, pure water, blood seawater, milk of magnesia among others.
Learn about neutral solution here https://brainly.com/question/21444245
#SPJ1
A 12.2 mL sample of liquid was found to have a mass of 10.4 g. Calculate the density of this liquid ( in g/mL).
Answers
Answer:
d=m/
Explanation:
d is density, m is mass, v is volume
Given: m =10.4g, v=12.2mL
substituting in equation,
d=10.4/ 12.2
d=0.8524g/mL
To learn more about density:
The density of the liquid is 0.852 g/mL.
To calculate the density of the liquid, we need to use the formula:
Density = Mass / Volume
Given that the mass of the liquid is 10.4 g and the volume is 12.2 mL, we can substitute these values into the formula:
Density = 10.4 g / 12.2 mL
Simplifying this expression, we find:
Density = 0.852 g/mL
Density is a physical property of a substance and is defined as the amount of mass per unit volume. In this case, the density tells us that for every milliliter of the liquid, there is 0.852 grams of mass. The units of grams per milliliter (g/mL) indicate that the density is a ratio of mass to volume.It is important to note that the density of a substance can vary with temperature, so this value is only valid under the conditions at which the measurement was made. Additionally, the density can provide valuable information about the identity of a substance, as different substances have different densities.
for such more questions on density
https://brainly.com/question/26364788
#SPJ8
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
9. Which type of reaction is shown by the equation below?
PAO10(s) + 6 H20 (1) ► 4 H3PO4(ay)
Synthesis
Combustion
оооо
o
Double replacement
Decomposition
Answers
A hot ballon with mass of 400 kilograms moves across the aky with 3,200 joules of kinetic energy. The velocity of the ballon is
Answers
Answer:
4 m/s
Explanation:
formula is v = (KE/.5m)^1/2
there is a calculator
https://www.calculatorsoup.com/calculators/physics/kinetic.php
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
How many grams of sodium hydroxide are needed to completely react with 50.0 grams of H2SO4?
Answers
Answer:
48.0 grams
Explanation:
The products of this reaction are nitrogen gas and water. How many grams of sodium hydroxide (NaOH) are needed to completely react with 50.0 grams of sulfuric acid (H2SO4) to form sodium sulfate (Na2SO4) and water? 2NaOH + H2SO4 → Na2SO4 + 2H2O ; 48.0 grams of NaOH are needed.
51.020 grams of sodium hydroxide is needed to completely react with 50 grams of sulfuric acid according to stoichiometry.
What is stoichiometry?It is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.
The reaction between sodium hydroxide and sulfuric acid is as follows,
2 NaOH + H₂SO₄\(\rightarrow\)Na₂SO₄+2 H₂O
From the reaction, it is clear that 2 oles of sodium hydroxide react with 1 mole of sulfuric acid to give products. According to molar mass concept,
100 g of sodium hydroxide gives 98 g of sulfuric acid
Therefore, 50 gram of sulfuric acid requires 50×100/98=51.020 g
Learn more about stoichiometry ,here:
https://brainly.com/question/28780091
#SPJ2
what is the mass of an iridium sample which absorbs 895 j of energy when its temperature increases by 12.3 C
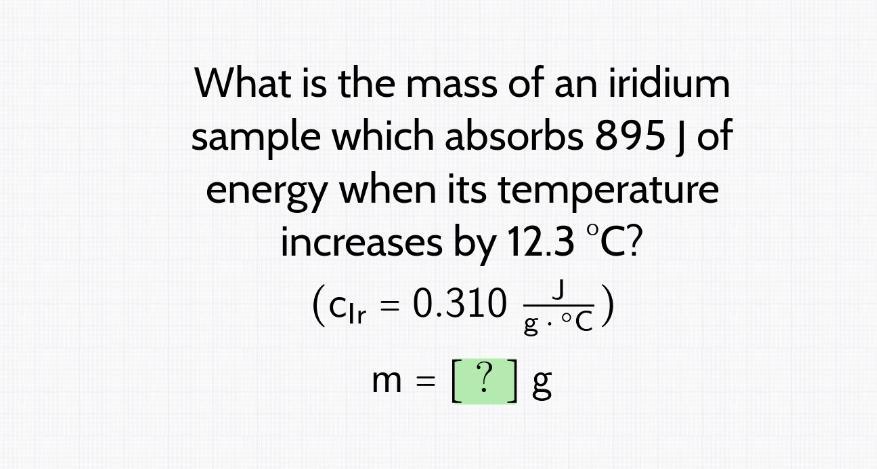
Answers
The mass of an iridium sample which absorbs 895J of energy when its temperature increases by 12.3°C is 234.72g.
How to calculate mass?The mass of a substance can be calculated using the following calorimetry equation:
Q = mc∆T
Where;
Q = quantity of heat absorbed or released (J)m = mass of substancec = specific heat capacity (J/g°C)∆T = temperature (°C)According to this question, an iridium sample absorbs 895J of energy when its temperature increases by 12.3°C. The mass can be calculated as follows:
895 = m × 0.310 × 12.3
895 = 3.813m
m = 234.72g
Therefore, 234.72g is the mass of the iridium sample.
Learn more about mass at: https://brainly.com/question/28992424
#SPJ1
I know I got this question wrong, but I am trying to find where I went wrong. I only have a problem with the Cooling Curves, not the Heating Curves.

Answers
The heat that is evolved is 8.77 kJ.
What is the heat that is released?We know that heat can neither be created nor destroyed but the heart can be transformed from one form to the other. We have been told here that the water was cooled from 115°C to -50°C. This heat would be evolved in steps as shown below;
1) H = 3 * 2.03 * (115 - 100)
H = 91.35 J
2) H = 3 * 2240
= 6720 J
3)H = 3 * 4.18 * (100 - 0)
= 1254 J
4) H = 3 * 334
= 1002 J
5) H = 3 * 2.09 * (-50 - 0)
H = -300 J
The total heat that is required;
= 91.35 J + 6720 J + 1254 J + 1002 J + (-300 J)
H = 8.77 kJ
Learn more about heat:https://brainly.com/question/13220736
#SPJ1
What amount of heat, in kJ, is required to vaporize 163.45 g of ethanol (C₂H₅OH)? (∆Hvap = 43.3 kJ/mol)
Answers
Answer:
Amount of heat required = 153.62 J
Explanation:
Given:
Mass = 163.45 g
∆Hvap = 43.3 kJ/mol
Molar mass C₂H₅OH = 46.07 g/mol
Find:
Amount of heat required
Computation:
Amount of heat required = Number of moles x Molar mass C₂H₅OH
Amount of heat required = [163.45/46.07][43.3]
Amount of heat required = 153.62 J
When testing for hydrogen gas in the laboratory a glowing splint is inserted into the gas which results in a pop sound. What is associated with the formation of the pop sound?
A. physical change
B. chemical change
C. exothermic reaction
D. endothermic reaction
Answers
Consider the following equilibrium:

Answers
The equilibrium concentration of PCl5 will increase PCl3 is added.
What is equilibrium?A reversible reaction is said to be in equilibrium when the rates of the forward and the backward reaction are equal.
According to a law of reaction, when a reaction is in equilibrium and one of the constraints that affect the rate of reaction is applied, the equilibrium will shift so as to annul the effects of the constraint introduced.
Thus, in this case, in order for more PCl5 (a reactant) to be formed, PCl3 (a product) would have to be added.
More on equilibrium can be found here: https://brainly.com/question/11114490
please help me and promise I will mark as brainiest I need it in ISEF please
how much energy does the vehicle hydrogen fuel cell need to break hydrogen into ions ?!
Answers
Answer: A typical hydrogen fuel cell produces 0.5 V to 0.8 V per cell. To increase the voltage individual cells can be connected in series.
7. *You have a 1.2 M solution of CaCl, that has a final volume of 0.050 L.
a. How many moles of CaCl₂ are generated in the reaction?
b. How many grams of CaCl₂ are generated in the reaction?
Answers
Answer:
A
Explanation:
solution contains 3.5 moles CaCl2/L solution . We need to convert moles CaCl2 to grams.
Hope this helps :)
The density of crystalline cl2 at 160K is 2.02g/cm3. calculate the molar volumes
Answers
The molar volume (Vm) is the volume occupied by one mole of a chemical element or chemical compound at standard temperature and pressure (STP). The molar volume of Cl₂ is 35.10 cm³/mol.
One mole of any gas has a defined volume when it is at a particular temperature and pressure. The relationship between molar mass and molar volume is direct and inverse, respectively.
Two gases have equal molecules if their volumes are the same at a given temperature and pressure. A gas's volume is directly proportional to the number of moles it contains. One mole of any gas takes up 22.4 L of space at STP.
The expression used to calculate molar volume is:
Molar volume = Molar mass / Density
The molar mass of Cl₂ = 70.906 g/mol
Molar volume = 70.906 / 2.02
Molar volume = 35.10 cm³/mol
To know more about molar volume, visit;
https://brainly.com/question/29884686
#SPJ3
the mass spectrum of an organic compound shows the relative abundances of m m to be 44.75% 44.75 % and m 1 m 1 to be 2.904%. 2.904 % . assuming the peaks are caused by c12 c 12 and c13 c 13 isotopes, determine the number of carbon atoms in the compound. the natural abundance of c12 c 12 is 98.93%, and the natural abundance of c13 c 13 is 1.07%. number of carbon atoms:
Answers
The compound contains approximately 0.1486.02210^23 carbon atoms, or about 8.9*10^22 carbon atoms. Thus, the number of carbon atoms in the compound is approximately 15.
Define molecular formula.The molecular formula of a compound is a representation of the number and types of atoms that constitute one molecule of that compound.
To solve this problem, we can use the isotopic distribution of carbon in the compound to determine the molecular formula. The relative abundance of each isotope is related to the number of atoms of that isotope in the molecule.
Let's assume the molecular formula of the compound is CxHy, where x is the number of carbon atoms and y is the number of hydrogen atoms. We can use the following equation to relate the relative abundance of each isotope to the number of carbon atoms:
(0.9893)x(0.4475) + (0.0107)x(0.02904) = 0.02904
Simplifying this equation, we get:
0.443x + 0.00031268x = 0.02904
0.44331268x = 0.02904
x = 0.06556/0.44331268
x = 0.148
Therefore, the compound contains approximately 0.1486.02210^23 carbon atoms, or about 8.9*10^22 carbon atoms. Thus, the number of carbon atoms in the compound is approximately 15.
Learn more about carbon atoms here:
https://brainly.com/question/13990654
#SPJ1
The number of carbon atoms in the compound can be determined by calculating the ratio of C12 to C13 isotopes present.
What is carbon atoms?Carbon atoms are the building blocks of life. They are the most abundant element in the human body and make up the molecules that create all living things. Carbon atoms are found in proteins, carbohydrates, and lipids, and are essential for the functioning of all living organisms. Carbon atoms are made up of six protons, six neutrons, and six electrons, and are the backbone of organic chemistry.
Since the relative abundances of C12 and C13 are 44.75% and 2.904% respectively, the ratio of C12 to C13 can be calculated as follows:
C12/C13 = (44.75/2.904) = 15.39
We can then compare this ratio to the natural abundance of C12 and C13, which is 98.93% and 1.07%, respectively.
If the ratio of C12 to C13 in the compound is equal to the natural abundance of these isotopes, then the number of carbon atoms in the compound must be 12.
C12/C13 = (98.93/1.07) = 92.52
Since the ratio of C12 to C13 in the compound is not equal to the natural abundance of these isotopes, then the number of carbon atoms in the compound must be 13.
To learn more about carbon atoms
https://brainly.com/question/27860158
#SPJ1
If the H3O is 4.950 x 10-12 what is the ph?
Answers
Answer:
pH = 11.3
Explanation:
From the question given above, the following data were obtained:
Concentration of hydronium ion [H₃O⁺] = 4.950×10¯¹² M
pH =.?
The pH of a solution is defined by the following equation:
pH = –Log [H₃O⁺]
Thus, with the above formula, we can obtain the pH of the solution as follow:
Concentration of hydronium ion [H₃O⁺] = 4.950×10¯¹² M
pH =.?
pH = –Log [H₃O⁺]
pH = –Log 4.950×10¯¹²
pH = 11.3
A gas has a pressure of 6.5 atm at a temperature of 500. K. At what temperature will the pressure be 4.8 atm?
Answers
To solve this question we have use Gay-Lussac's Law:
\(\frac{P1}{T1}=\frac{P2}{T2}\)Where P1 and P2 are the pressures and T1 and T2 are the temperatures.
In this case, we know the values of P1, P2 and T1 and we have to find T2:
\(\begin{gathered} T2=T1\cdot\frac{P2}{P1} \\ T2=500K\cdot\frac{4.8atm}{6.5atm} \\ T2=369.23K \end{gathered}\)The pressure will be 4.8atm at 369.23K.
Which formula shows the correct ratio of ions in thecompound formed by each pair of elements? Why?Element Q. Element R 7. Aluminum Sulfur8. Potassium oxygen 9. lithium chloride 10. Strontium. bromine A) QR (B) QR2 (C) QR (D) Q2 R3
Answers
7) or A) QR
Al and S => QR = AlS
This is not correct because the Oxidation state of Al is +3 and for S should be -3 too, but S doesn't have a number 3 as oxidation state.
8) or B) QR2=> KO2
It's incorrect, It should be K2O
9) or C) QR => LiCl
This is correct, here the charges are +1 for Li and -1 for Cl
LiCl => Li+1 + Cl-1
10) or D)Q2R3 => Sr2Br3
This isn't correct, Oxidation state for Sr is +2, not +3
Answer: 9) or C) LiCl
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
All the questions to The power of advertising commonlit answers.
Answers
What is the statistical rule of thumb to determine adequate sample size? Question 33 options: A statistical power level of 60 percent A statistical power level of 50 percent A statistical power level of 10 percent A statistical power level of 90 percent A statistical power level of 80 percent
Answers
The statistical rule of thumb to determine an adequate sample size include the following: E. A statistical power level of 80 percent.
What is sampling?In Mathematics and Statistics, sampling can be defined as a process that is typically used for the collection or selection of data (physical objects, observations, or individuals) from a larger statistical population, especially by using specific procedures and processes.
What is a sample proportion?In Mathematics and Statistics, a sample proportion can be defined as the proportion of individuals in a sample that have a specified characteristic or trait.
Mathematically, the sample proportion of a sample can be calculated by using this formula:
\(\hat{p} = \frac{x}{n}\)
Where:
x represents the number of individuals with a specified characteristic.n represents the total number of individuals in a sample.In Mathematics and Statistics, a statistical power level of 80 percent (80%) is a statistical rule of thumb that is generally used in determining an adequate sample size.
Read more on sample proportion here: brainly.com/question/23838641
#SPJ1
write down the formulas and predict the products. Name and balance the equation iron(ii) bromide + Barium Chloride
Answers
The formula for iron(II) bromide is FeBr2, while the formula for barium chloride is BaCl2. The products of the reaction are barium bromide (BaBr2) and iron(II) chloride (FeCl2).
What is Balanced Equation ?
A balanced chemical equation is a symbolic representation of a chemical reaction that shows the relative numbers of reactant and product molecules or ions involved. In a balanced equation, the number of atoms of each element must be the same on both the reactant and product sides, in order to obey the law of conservation of mass.
The balanced chemical equation for the reaction between iron(II) bromide and barium chloride is:
FeBr2 + BaCl2 → BaBr2 + FeCl2
In this reaction, the iron(II) ion (Fe2+) in iron(II) bromide (FeBr2) is replaced by the barium ion (Ba2+) from barium chloride (BaCl2), forming barium bromide (BaBr2) and iron(II) chloride (FeCl2). The balanced equation shows that two bromide ions (Br-) and two chloride ions (Cl-) are involved in the reaction, which ensures that the equation is balanced in terms of both mass and charge.
Learn more about Balanced Equation from given link
https://brainly.com/question/11904811
#SPJ1
Write a paragraph to name and describe two renewable energy sources used for electricity production and examine the advantages and disadvantages. needs to be written in a POWTREE paragraph
Answers
Answer:
Renewable energy describes a collection of energy technologies, i.e., solar, wind, geothermal derived from sources that are never-ending and can be replenished time after time. Most countries across the world heavily depend on fossil fuels (oil, coal and natural gas) as sources of energy to power their economies. Renewable sources are renewable, sustainable, abundant and environmentally friendly. Unlike fossil fuels, they are not going to expire soon as they are constantly replenished.
Fossil fuels are non-renewable forms of energy, meaning they utilize limited resources that will ultimately deplete, hence, driving up overall energy costs. These countries have responded to the threat by stepping up campaigns to embrace renewable forms of energy like solar and wind.
Explanation:
pliz mark me branliest pliz
NEED HELP ON QUESTION ASAP! !
If answer is correct I'll rate you five stars a thanks and maybe even brainliest!
Please can you explain what this paragraph is trying to say. Also what does it mean in the sentence 'the difference in charge across the battery provides push for current' and what is the difference in charge.
Here's paragraph I need to have a simple definition of:
A high waterfall is also like a large voltage. It will transfer a lot of energy to the water (charge), making the river flow very fast (a large current) the difference in height makes the river flow. In a circuit , the difference in charge across battery provides push for the current.
Answers
A waterfall is a river's water falling rapidly to the ground. It is created in a river's upper course where there are high mountains.
Thus, Many waterfalls are transient and only occur during rainstorms because of their location in the landscape, where they are often over bedrock and fed by a small contributing region.
For general knowledge, we are listing the "Top 10 Highest Waterfalls in India" here. The Kunchikal Falls are the second-highest waterfall in Asia and the highest in all of India.
The waterfall, which is close to Agumbe in Karnataka's Shimoga district, has a height of 1,493 feet. The Varahi River creates the biggest waterfall. The sole permanent rain forest research is located in Agumbe Valley, one of India's most heavily rained-on regions.
Thus, A waterfall is a river's water falling rapidly to the ground. It is created in a river's upper course where there are high mountains.
Learn more about Waterfall, refer to the link:
https://brainly.com/question/30309599
#SPJ1
Based on the reaction, identify the products. BeF2 + Mg → MgF2 + What type of reaction does this represent?
Answers
Answer:
Be replacement
Explanation:
srry they delted my answer before:( someone from brainly all i did was added a link for a quizlet that would help you.. :(
Answer:
Be
Replacement
Explanation:

Instead of collecting gas with a syringe, another method involves: • Submerging the evidence in a special agent • Placing a strip of charcoal in the airtight container • Using a tiny vacuum • All of the above
Answers
Instead of collecting gas with a syringe, another method involves Using a tiny vacuum.
what is gas ?One of the state of matter is Gas in which the particles are far apart, fast-moving and not organized in any particular way.
These are the substances which exist in the gaseous state, one of the three fundamental states of matter, are highly compressible and feature very large intermolecular distances.
The gaseous state is very small attractive forces between the gas particles which is separated from each other by relatively greater distances when compared to liquids and solids.
To learn more about gas , refer to the link:
https://brainly.com/question/3450728
#SPJ2
What limitations occurs for chalk in vinegar chemistry pd lab experiment?
Also the precautions to take
Need this asap!!
Answers
Answer:
When conducting a chemistry lab experiment using chalk (calcium carbonate) in vinegar (acetic acid), there are several limitations and precautions to be aware of:
Limitations of chalk in vinegar chemistry experiment:
Reaction rate: The reaction between chalk and vinegar is relatively slow, which may require a longer observation period or higher concentration of vinegar to observe significant changes within a reasonable time frame.
Solubility: Chalk may not dissolve completely in vinegar, resulting in incomplete reaction or difficulty in obtaining accurate results.
Product formation: The reaction between chalk and vinegar produces carbon dioxide gas, water, and calcium acetate. The carbon dioxide gas may escape into the atmosphere, leading to loss of product and inaccurate measurements.
pH: Chalk is a basic substance, and the reaction with vinegar, which is acidic, may result in neutralization, leading to a decrease in the overall acidity of the reaction mixture.
Precautions to take in chalk in vinegar chemistry experiment:
Ventilation: The reaction between chalk and vinegar produces carbon dioxide gas, which can displace air and potentially cause asphyxiation in a closed or poorly ventilated area. Conduct the experiment in a well-ventilated area or under a fume hood to ensure adequate air circulation.
Eye and skin protection: Vinegar is an acid and can cause skin and eye irritation. Wear appropriate personal protective equipment (PPE), such as gloves and goggles, to protect yourself from contact with vinegar or any other chemicals used in the experiment.
Chemical handling: Handle the chemicals, including chalk and vinegar, with care, following proper lab safety protocols. Avoid ingestion, inhalation, or direct contact with the chemicals, and dispose of them properly according to local regulations.
Accuracy in measurements: Use calibrated and accurate measuring tools, such as graduated cylinders or burettes, to measure the amount of chalk, vinegar, and other reagents accurately. This will ensure the reliability and accuracy of the experimental results.
Observations: Make careful and detailed observations during the experiment, noting any changes in appearance, gas evolution, or other relevant observations. Take measurements at appropriate intervals and record the data accurately for analysis and interpretation.
It is important to follow good laboratory practices, including proper chemical handling, accurate measurements, and cautious observations, to ensure safe and reliable results in a chalk in vinegar chemistry lab experiment. Consult with a qualified instructor or supervisor for specific guidelines and precautions related to your experiment.
There are 3 reactions of Calcium Carbonate, CaCO₃, that can be formed in this particular problem:
Reaction 1: Calculate the ΔH₁
Reaction 2: ΔH₂ = -635.1 kJ
Reaction 3: ΔH₃ = 178.3 kJ

Answers
ΔH₁ = ΔH₂ - ΔH₃
ΔH₁ = -635.1-(178.3) KJ
ΔH₁ = -813.4 KJ
Of the following regions of the electromagnetic spectrum, which one has the shortest wavelength?
a.
gamma rays
b.
infrared
c.
radio waves
d.
X rays
e.
microwaves
f.
ultraviolet
Answers
Answer:
A ---->gamma ray
Explanation:
Gamma rays have the highest frequencies among all electromagnetic waves and therefore have the shortest wavelengths.