Assuming both graduated cylinders are holding water at room temperature, which cylinder has more thermal energy?
A. The graduated cylinder with more water has more thermal energy because it is holding more water particles.
B. The graduated cylinder with more water has more thermal energy because the particles are warming through conduction.
C. The graduated cylinder with less water has more thermal energy because and less average kinetic energy.
D. Neither graduated cylinder has thermal energy because the water has not been heated.
Answers
Answer:
D
Explanation:
bothe cylinders are at room temperature so no cylinder has more themal energy. That crosses off the answers A B and C.
Related Questions
the SI unit for forces is it a newton, force, unbalanced forces, balanced forces, net forces pick on I need you help
Answers
Answer: The SI unit for force is Newtons (N).
fill in the blanks: ozone is made of __________ and is broken down by __________.
a. hydrogen, zinc
b. sodium, molybdenum
c. oxygen, chlorine
d. chlorine, fluorine
Answers
Please mark brainliest I need three more!!!!!!!!
_____ is a disaccharide important in the formation of alcoholic beverages.
Answers
Maltose is a disaccharide important in the formation of alcoholic beverages.
Maltose:
Maltose, also known as maltobiose or maltose, is a disaccharide formed from two glucose units linked by α(1 → 4) bonds. In isomeric isomaltose, the two glucose molecules are linked by an α(1 → 6) bond. Maltose is both members of the amylose homologous family, which is the major structural motif in starch. When beta-amylase breaks down starch, it removes two units of glucose at a time to form maltose. An example of this reaction can be found in germinating seeds, which is why malt is named after it. Unlike sucrose, it is a reducing sugar.
Also known as malt sugar, it is made up of two glucose molecules bonded together. It is an important disaccharide in the formation of alcoholic beverages.
Alcoholic Beverage:
Alcoholic beverages (also called alcoholic beverages, adult beverages, or beverages) are beverages that contain ethanol, a type of alcohol that acts like a drug and is made by fermenting grains, fruits, or other sources of sugar. Drinking alcoholic beverages, often referred to as "drinking", plays an important social role in many cultures. Most countries have laws regulating the production, sale and consumption of alcoholic beverages.
Regulations may require the alcohol percentage (alcohol or proof) to be displayed and warning labels to be used. Alcoholic beverages are legal in most countries around the world, although in some countries such activities are outright banned. The global liquor industry passed $1 trillion in 2018.
Learn more about Maltose:
https://brainly.com/question/19755762
#SPJ4
Answer:
Ethanol is a disaccharide important in the formation of alcoholic beverages. Ethanol is produced by yeast during fermentation when sugar is converted to alcohol. This process involves the breaking down of carbohydrates, such as glucose and fructose, into simple sugars, which are then converted into ethanol.
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
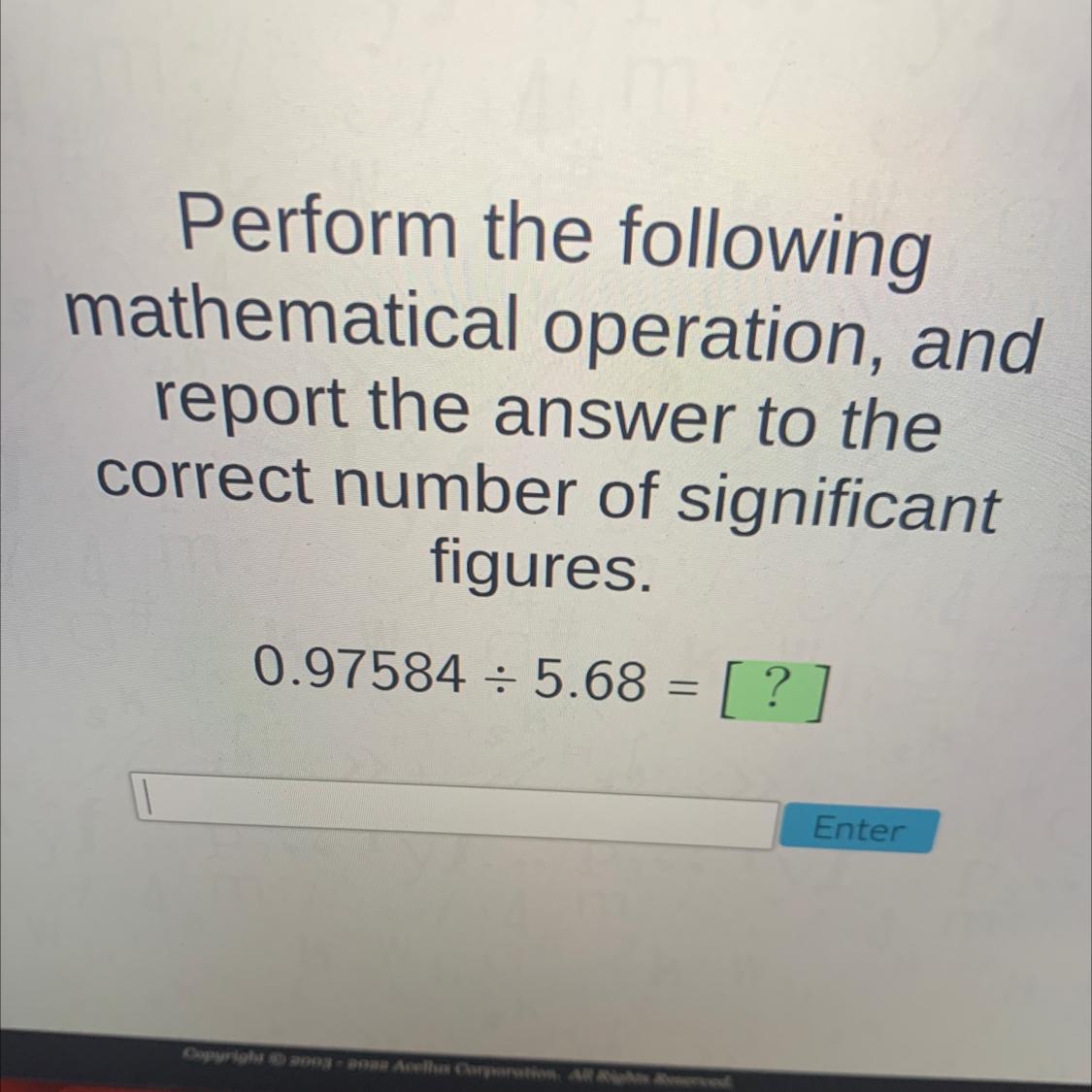
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Use the chemical reaction to answer the question.
4Fe+3O2→2Fe2O3
What happens to atoms of Fe and O2 during the reaction?
(1 point)
Responses
They gain mass.
They are rearranged.
They are lost.
They maintain their properties.
Answers
Answer:
hair redox reaction occurs so that is why they rearrange is well as they loss and gain of electron
Add. Write your answer as a mixed number in simplest form. 3(3)/(8)+6(1)/(2)
Answers
The sum of 3(3)/(8) and 6(1)/(2) is 5(5)/(8), which is a mixed number representing 5 whole units and 5/8 of another unit.
To find the sum of mixed numbers, we need to add the whole numbers and the fractions separately. Add the whole numbers: 3 + 6 = 9
Add the fractions: For the fractions 3/(8) and 1/(2), we need to find a common denominator. The least common multiple of 8 and 2 is 8.
Converting 3/(8) to have a denominator of 8:
3/(8) = (3 x 1)/(8 x 1) = 3/(8)
Converting 1/(2) to have a denominator of 8:
1/(2) = (1 x 4)/(2 x 4) = 4/(8)
Now, we can add the fractions:
3/(8) + 4/(8) = (3 + 4)/(8) = 7/(8)
Combine the whole numbers and fractions:
The whole numbers sum was 9 and the fractions sum was 7/(8). We can write this as a mixed number by dividing the numerator (7) by the denominator (8). The quotient is 0 with a remainder of 7. Therefore, the mixed number is 0(7)/(8), which can be simplified to 7/(8).
Therefore, sum of 3(3)/(8) and 6(1)/(2) is 5(5)/(8).
Learn more about mixed number
brainly.com/question/11553376
#SPJ11.
which compound in each of the following pairs of ionic substances has the most exothermic lattice energy? justify your answers. mgo, bas
Answers
The compounds of ionic substances with the most exothermic lattice energy are:
LiF, LiCl Mg(OH) 2, MgO Fe(OH) 2, Fe(OH) 3Correct answer: letters B, C y D.
This is because these ionic substances have the most exothermic lattice energy because they form ionic bonds, which are very strong, and these three alkali metals are in the first row of the periodic table.
What are the compounds with the highest exothermic lattice energy?The compounds of ionic substances with the most exothermic lattice energy are those in which the ions have the largest difference in electronegativity.
The lattice energy of an ionic substance is the energy required to completely separate the ions in the solid state. The compound with the most exothermic lattice energy is the one that is the most stable. In general, the larger the ionic radius, the more exothermic the lattice energy.
Which compound in each of the following pairs of ionic substances has the most exothermic lattice energy?
Group of answer choices:
a) NaCl, KCl
b) LiF, LiCl
c) Mg(OH) 2, MgO
d) Fe(OH) 2, Fe(OH) 3
e) NaCl, Na 2
f) MgO, BaS
Learn more about exothermic grid energy:
https://brainly.com/question/2868767
#SPJ4
Explain whether changing the ratio of baking soda and vinegar changes the amount of carbon dioxide produced. Include the evidence you used to reach your conclusion.
Answers
On changing the ratio of baking soda and vinegar changes the amount of carbon dioxide produced is true, because each species will depends on both reactants.
What is chemical reaction?Chemical reactions are those reactions in which reactants are mixed with each other for the formation of new product.
Chemical reaction between baking soda and vinegar is shown below in the attached image in which the formation of carbon dioxide, water and sodium acetate takes place. So the formation of carbon dioxide depends on the baking soda and baking soda reacts with vinegar to form sodium acetate so change in the ratio will changes the amount of carbon dioxide.
Hence on changing the ratio of reactants and vinegar changes the amount of carbon dioxide.
To know more about chemical reactions, visit the below link:
https://brainly.com/question/26018275

When the oil urushiol from the poison ivy plant is absorbed through the skin, it oxidizes and forms a substance called quinone. Quinone reacts with skin proteins creating a complex that now triggers an immune response. Quinone is an example of a(n) __________. rev: 02_10_2018_QC_117574
Answers
Quinone is an example of a T cell immune system.
What is Immune system?These refers to the cells and organs present in organisms which are responsible in providing resistance to germs and infections.
T cells form part of the immune system and provide adaptive immune response through attacking foreign bodies in the body. Quinone reacting with skin proteins and creating a complex that now triggers an immune response depicts the defense mechanism of T cells.
Read more about Immune system here https://brainly.com/question/4457677
Select all of the following that are components of nucleotides. a. nitrogen-containing base b. hydrocarbon tail attached to a polar head c. glycerol d. sugar e. phosphorus-containing groups
Answers
Answer:
A and E
Explanation:
with Adenine ,thymine , cytosine and guanine
Calculate:for each object, substitute the values you know into the gravitational potential energy equation to solve for weight. Record each object's weight in the fourth column.
Answers
Answere:No sé esto jeje lo siento no soy tonta pero simplemente no sé esto
Explanation:
What is the charge on the complex ion in Ca2[Fe(CN)6]? Is it 1-,2-,2+,3-, or 4-?
Answers
The charge on the complex ion in Ca2[Fe(CN)6] is 4-. To understand this, let's break down the complex ion.
The [Fe(CN)6] unit is a hexacyanoferrate(II) ion, which means that the iron in the center has a +2 charge. Each cyanide ion (CN-) has a -1 charge, so the total charge of the [Fe(CN)6] unit is -6. When this unit is coordinated with the Ca2+ ion, which has a 2+ charge, the overall charge of the complex ion is -4.
Therefore, the correct answer is 4-. It's important to note that determining the charge of a complex ion can be complex and requires an understanding of coordination chemistry and oxidation states.
To know more about complex ion visit:
https://brainly.com/question/28304376
#SPJ11
A gas has a volume of 62.65 L at stp. At what temperture in C would the volume of the gas be 78.31 at a pressure of 612 mm hg
Answers
Answer:
1.79°C
Explanation:
Applying,
PV/T = P'V'/T'................. Equation 1
Where P = Initial presssure, V = Initial volume, T = Initial temperature, P' = Final pressure, V' = Final volume, T' = Final Temperature.
make T' the subject of the equation
T' = P'V'T/PV................ Equation 2
From the question,
Given: P = 760 mmHg (Standard pressure), T = 273 K (Standard temperature), V = 62.65 L, P' = 612 mmHg, V' = 78.31
Substitute these values into equation 2
T' = (612×78.31×273)/(760×62.65)
T' = 274.79 K
T' = (274.79-273) °C
T' = 1.79°C
Predict the product(s) and write a balanced equation for each of the following redox reactions:
(c) Sulfur dioxide + oxygen →
Answers
Sulfur dioxide can be further oxidized by oxygen to sulfur trioxide:
Balanced equation:
2 SO2 + O 2 → 2 SO3
All redox reactions can be divided into two categories: reduction reactions and oxidation reactions. The oxidation-reduction reaction, commonly referred to as the redox reaction, always comprises concurrent oxidation and reduction reactions.
Sulfur dioxide dissolves in water, forming sulfurous acid.
What happens when sulfur dioxide dissolves in water?when it dissolves in water, it turns into sulfuric acid, a corrosive liquid. Moist sulfur dioxide is quite corrosive because it gradually converts to sulfuric acid . Containers may explode in the heat of a fire or burst and release irritating, deadly sulfur dioxide.
Which of the following fuel combustion byproducts dissolves in rainfall?Acid Bis created when some byproducts of fuel combustion dissolve in rainwater. Acid rain and sulfur dioxide. Sulfur impurities can be found in a lot of fossil fuels. Sulfur in these fuels is oxidized during combustion to produce sulfur dioxide. After dissolving in raindrops of water, the sulfur dioxide becomes sulfuric acid.
Learn more about Sulfur and oxygen:
https://brainly.com/question/13469437
#SPJ4
A circuit that offers 40 ohms resistance, is connected to a 60 v supply .how much current can it generate?
A. 120A
B. 120V
C. 3A
D. 3V
Answers
The amount of current generated, given that the circuit offers 40 ohms resistance, and is connected to a 60 V supply is 1.5 A
How do I determine the current generated by the circuit?The following data were obtained from the question given above:
Resistance (R) = 40 ohmsVoltage (V) = 60 V Current (I) =?Ohm's law states as follow:
Voltage (V) = Current (I) × resistance (R)
Using the above formula, the current generated by the circuit can be obtained as follow:
Voltage (V) = Current (I) × resistance (R)
60 = Current × 40
Divide both sides by 40
Current = 60 / 40
Current = 1.5 A
Thus, the current generated in the circuit is 1.5 A (None of the options are correct)
Learn more about current:
https://brainly.com/question/23754329
#SPJ1
The percentage yield of a reaction compares to the actual yield with the ... yield. What completes the sentence?
Answers
Answer:
infinite yield woth sentence
Explanation:
this comple3tes sentence bc infinite yield
it does es
Of the following elements aluminum, magnesium, aluminum, silicon, and sodium which has the smallest atomic radius? Explain your answer.
Answers
Answer:
Sodium
Explanation:
Radius grows as you go down and to the right on the ptable.
If 30 g of a drug is dissolved in 150 mL of a solvent having a specific gravity of 1.40, what is the percentage strength (%w/w) of the drug solution?
Answers
the percentage strength (%w/w) of the drug solution is 12.5%.To determine the percentage strength (%w/w) of the drug solution, we need to first calculate the weight of the solvent in the solution. We can do this using the specific gravity of the solvent, which tells us how much denser the solvent is compared to water.
To determine the percentage strength (%w/w) of the drug solution, we need to first calculate the weight of the solvent in the solution. We can do this using the specific gravity of the solvent, which tells us how much denser the solvent is compared to water.
Density of water = 1 g/mL
Density of solvent = 1.40 g/mL
Therefore, the weight of the solvent in 150 mL of the solution is:
Weight of solvent = Volume x Density = 150 mL x 1.40 g/mL = 210 g
Now, to find the percentage strength (%w/w) of the drug solution, we need to divide the weight of the drug by the total weight of the solution (drug + solvent) and multiply by 100.
Weight of drug = 30 g
Total weight of solution = 30 g + 210 g = 240 g
%w/w of drug solution = (Weight of drug / Total weight of solution) x 100
%w/w of drug solution = (30 g / 240 g) x 100
%w/w of drug solution = 12.5%
Therefore, the percentage strength (%w/w) of the drug solution is 12.5%.
To determine the percentage strength (%w/w) of the drug solution with 30 g of drug dissolved in 150 mL of a solvent with a specific gravity of 1.40, follow these steps:
1. Calculate the mass of the solvent:
Mass of solvent = Volume of solvent × Specific gravity
Mass of solvent = 150 mL × 1.40 g/mL = 210 g
2. Calculate the total mass of the solution:
Total mass = Mass of drug + Mass of solvent
Total mass = 30 g (drug) + 210 g (solvent) = 240 g
3. Calculate the percentage strength (%w/w):
Percentage strength = (Mass of drug / Total mass) × 100
Percentage strength = (30 g / 240 g) × 100 = 12.5 %
Therefore, the percentage strength (%w/w) of the drug solution is 12.5%.
To know more about percentage strength, visit
https://brainly.com/question/17130362
#SPJ11
Name these compounds according to IUPAC. (ASAPP)

Answers
The chemical's IUPAC names of the given compound are propanal and acetone.
What is IUPAC naming?IUPAC is the international union of practical and applied chemistry. It set rules for standard naming of the compound.
Propanal, or propanaldehyde, is an aldehyde that has one double bond and one oxygen atom.
Acetone is a kind of ketone. Ketones have the functional group R2CO.
Thus, chemical's IUPAC names of the given compound are propanal and acetone.
Learn more about IUPAC naming
https://brainly.com/question/16631447
#SPJ1
numerical Through the two ends of glass-tube of lenght 2 meters, hydrogen chloride and ammonia gases are allowed to enter.At what distance ammonium chloride will first appear?
Answers
Answer:
81cm is the answer.
Explanation:
Let X be the length from the HCl end, and therefore, (2-x) from the other end.
For X length, rate of diffusion is :
dx/dt=P A/(Square root of 36.5T
d(2−x)/dt=PA/(square root of 17 T
Dividing both we get;
X/(2−x)= square root of 36.5/17
X=2/2.466=0.81 or81cm
Following is a list of properties of a sample of solid sulfur: i. Brittle, crystalline solid. ii. Melting point of 113°C. iii. Density of 2.1 g/cm3. iv. Combines with oxygen to form sulfur dioxide. Which, if any, of these properties would be the same for one single atom of sulfur obtained from the sample? a. i and ii only.
b. iii and iv only.
c. iv only.
d. All of these properties would be the same. e. None of these properties would be the same.
Answers
"Combines with oxygen to form sulfur dioxide" is the property which is same for one single atom of sulfur obtained from the sample. Therefore, the correct option is option C.
What is sulfur?Sulfur (or sulphur in British English) is indeed a chemical element with the atomic number 16 and the symbol S. It's plentiful, multivalent, and nonmetallic. Sulfur atoms are connected cyclic achieve this by creating molecules with the chemical formula S8 under normal circumstances. At normal temperature, elemental sulfur is a brilliant yellow, crystalline solid.
Sulfur is indeed the tenth most plentiful element in the universe by mass as well as the fifth most prevalent on Earth. Sulfur on Earth is often found in sulfide and sulfate minerals, while it is occasionally discovered in pure, natural form. "Combines with oxygen to form sulfur dioxide" is the property which is same for one single atom of sulfur obtained from the sample.
Therefore, the correct option is option C.
To learn more about sulfur, here:
https://brainly.com/question/11612251
#SPJ1
All of the following are involved in transcription except
a) polymerase.
b) primer.
c) promoter.
d) sigma factor.
e) uracil.
Answers
polymerases
Promoter
Uracil
Sigma factor
a rock which has a mass of 60 grams and a volume of 20ml whats the density
Answers
Answer:0.67
Explanation:
vWhich statements explain why rocks weather at different rates? Check all that apply.
Softer, porous, or more permeable rocks weather faster than harder rocks.
Rocks in warmer climates weather faster than rocks in colder climates.
Rocks with minerals that dissolve slowly in water will weather faster than other rocks.
The more water present, the faster the rate of weathering.
Rocks that are more permeable are more resistant to weathering.
Answers
The correct option is A. Softer, porous, or more permeable rocks weather faster than harder rocks.
What are the different types of rocks?Rocks are of different types such as metamorphic, igneous, and sedimentary rocks.
Rocks that are more pliable, porous, or permeable weather more quickly than tougher rocks. Rocks weather more quickly in hot regions than in chilly ones.
In comparison to other rocks, rocks having minerals that break down slowly in water will weather more quickly. The rate of weathering increases with the amount of water present.
Therefore, The correct option is A. Softer, porous, or more permeable rocks weather faster than harder rocks.
To learn more about rocks, refer to the link:
https://brainly.com/question/19930528
#SPJ1
pls help what’s the answer?

Answers
1.calculate the ph of the solution after 15.0 ml of 0.100 m naoh has been added to the 25.0 ml of 0.100 m hcl solution. 2.calculate ph and [oh-] of a 5 x 10-3m hclo4 solution. ph
Answers
After mixing 15.0 mL of 0.100 M NaOH with 25.0 mL of 0.100 M HCl, the resulting solution will have a pH of approximately 1.20.
The pH of a 5 x 10^(-3) M HClO4 solution is approximately 2.30, and the [OH-] (hydroxide ion concentration) can be calculated as follows:
[OH-] = 10^(-pOH)
Since pOH + pH = 14, we can calculate the pOH by subtracting the pH from 14, giving us a pOH of approximately 11.70. Therefore,
[OH-] = 10^(-11.70)
To learn more about pH and [oh-] from the given link
brainly.com/question/13557815
#SPJ4
Which elements tend to have the largest atomic radius in their periods?.
Answers
Elements on the left side of a period tend to have the largest atomic radius within their periods.
In a period (horizontal row) of the periodic table, atomic radius generally decreases from left to right. This trend occurs because, within a period, the number of protons in the nucleus increases as we move from left to right, while the shielding effect remains relatively constant. The increasing positive charge of the nucleus attracts the electrons more strongly, resulting in a smaller atomic radius.
However, there are some exceptions to this trend, particularly with the transition metals. Within a transition metal period, the atomic radius may remain relatively constant or slightly decrease across the period due to the complex electron arrangements and the presence of additional electron shells.
That being said, the elements on the left side of a period (closer to the alkali metals and alkaline earth metals) tend to have the largest atomic radii within their respective periods. This is because they have fewer protons in the nucleus and more electron shells, which result in weaker attraction between the electrons and the nucleus. Examples of elements with relatively larger atomic radii in their periods include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), and cesium (Cs).
It's important to note that while this trend generally holds true, there can be variations due to specific electron configurations and other factors. Therefore, it's always best to consult the periodic table for precise atomic radius values and trends.
learn more about atomic radius here:
https://brainly.com/question/13963619
#SPJ11
a 30.00-ml sample of 0.125 m hcooh is being titrated with 0.175 m naoh. what is the ph after 30.0 ml of naoh has been added? ka of hcooh
Answers
The pH after 30.0 ml of NaOH has been added is 2.18.
To find the pH after 30.0 ml of 0.175 M NaOH has been added to a 30.00 ml sample of 0.125 M HCOOH, you need to use the balanced chemical equation for the reaction between HCOOH and NaOH:
HCOOH + NaOH → NaCOOH + H2O
This equation shows that 1 mole of HCOOH reacts with 1 mole of NaOH, so the number of moles of NaOH added to the HCOOH solution is:
n(NaOH) = C(NaOH) x V(NaOH) = 0.175 mol/L x 0.0300 L = 0.00525 mol
Since the stoichiometry of the reaction is 1:1, this means that 0.00525 mol of HCOOH has been neutralized by the NaOH. The remaining amount of HCOOH is:
n(HCOOH) = C(HCOOH) x V(HCOOH) - n(NaOH) = 0.125 mol/L x 0.0300 L - 0.00525 mol = 0.00225 mol
Now you can use the Ka expression for HCOOH to find the concentration of H+ ions:
Ka = [H+][COO-]/[HCOOH] = 1.8 x 10^-4
[H+][0.00225]/[0.1225 - 0.00525] = 1.8 x 10^-4
[H+] = 0.00659 M
pH = -log[H+] = 2.18
To learn more about balanced chemical equation click here
brainly.com/question/15052184
#SPJ11
All chemical reactions obey the law of conservation of mass.
Give
the reason why the reaction between hydrochloric acid and calcium carbonate appears not to show conservation of mass.
Answers
The reason the reaction between hydrochloric acid and calcium carbonate appears not to obey the law of conservation of mass is the evolution of carbon dioxide gas.
What is the law of conservation of mass?The law states that in every chemical reaction, the mass of the participating atom is conserved but the atoms themselves can change forms.
Thus, for every atom, the mass before the reaction must also be equal to the mass after the reaction.
So, if two substances A (1 g) and B (1 g) react, the products should weigh: 1 + 1 = 2 g.
Now, in the case of the reaction between hydrochloric acid and calcium carbonate, something happens.
\(CaCO_3 + 2HCl -- > CaCl_2 + H_2O + CO_2\)
One of the products formed is carbon dioxide gas. Unless the reaction vessel is sealed, the gas will escape and the weight of the products left will be less that the reactants.
More on the law of conservation of mass can be found here: https://brainly.com/question/28711001
#SPJ1
If you have 10 protons and 11 neutrons, in a electrically neutral atom, How many electrons would you have?
Answers
Answer:
Well, you'd have 1 electron more than protons (since there are 10 protons and 11 electrons). So the previously neutral ion has now become an ion with a -1 charge