Assume that the pendulum clock shown in the photograph is a closed
system
Which statement describes evidence that the pendulum's energy is conserved
even as it eventually stops moving?
A. Energy must be added to the pendulum to keep it from slowing
down
B. Some of the clock's matter is transformed into new energy
C. The amount of each type of energy in the system can be
measured
D. All its mechanical energy is transformed into other forms of
energy

Answers
Answer:
Explanation:
d
Answer:
D. All it’s mechanical energy is transformed into other forms of energy.
Explanation:
I got it right
Related Questions
please answer asap!
Which statement about the energy involved in nuclear binding energy is true?
a.It is the name given to the combination of strong and weak nuclear forces.
b.It is the energy that holds atoms together in a molecule.
c.It is the energy that binds the nucleus to the rest of the atom.
d.It is created when particles drift far apart.
Answers
It is the name given to the combination of strong and weak nuclear forces.
What is the true statement?We know that the nucleus of the atom is the place that houses the greatest amount of energy. We could obtain the highest energy by ripping open the nucleus of an atom. There is a huge energy that holds the particles in the nucleus together and this called the nuclear binding energy.
This energy must also be required to break the nucleus as is the case in the operation of the atomic bond and in nuclear reactors. The true statement about the nuclear energy is that; It is the name given to the combination of strong and weak nuclear forces.
Learn kore about nuclear energy:https://brainly.com/question/2240073
#SPJ1
If 125 mL of 0.56 MH2SO4, is diluted to a volume of 0.250 L, what is the molarity of the resulting solution?
Answers
Answer:
0.28M H2SO4
Explanation:
The molarity of a solution is defined as the ratio between the moles of the solute (In this case, H2SO4) and its volume in liters.
In the problem, the volume of the solution is increased from 125mL to 250mL. That means the solution is diluted:
250mL / 125mL = 2 times.
As the solution is diluted 2 times, the concentration is decreased in 2, that is:
0.56M / 2 =
0.28M H2SO41.Mitch weighs out 67 grams of potassium (K) to make a buffer. How many moles of potassium did Dr. Hellman weigh out?
2.Which statement is NOT true about a reaction rate?
Group of answer choices
Increases with increase in reactant concentration
Increases with increasing temperature
Is the speed at which product is formed
Is the rate at which reactant is used up
All of the answers are true
3.Which statement is NOT true about a catalyst?
Group of answer choices
Are not used up during a reaction
Increases the rate of the reaction
Lowers the energy of activation
Biological catalysts are called enzymes
Are used up during a reaction
Answers
Answer:
1. 1.72 moles of potassium.
2. All of the answers are true
3. Are used up during a reaction
Explanation:
Recall that the number of moles is obtained from;
Number of moles= Mass of potassium/ molar mass of potassium
Mass of potassium= 67 g
Molar mass of potassium= 39 gmol-1
Number of moles of K= 67 g/ 39 gmol-1
Number of moles = 1.72 moles of potassium.
2. When we look at all the options, we will realize that all the options are true. The rate of reaction doubles for each 10°C rise in temperature, increasing reactant concentration increases particle collision and ultimately increases the rate of reaction. Rate of reaction deals with rate of disappearance of reactants or rate of appearance of products.
3. Catalysts remain unchanged in a chemical reaction because they do not actually participate in the reaction. Hence they are not used up in any chemical reaction.
A 23. 6 g sample of Na3PO4 (molar mass 163. 94 g/mol) is dissolved in enough water to produce
750. ML of solution
Calculate the concentration of Nations in solution.
Write your answer using three significant figures.
Answers
The concentration of sodium ion, Na⁺ in the solution, given that 23.6 grams of Na₃PO₄ is dissolved in 750 mL of water is 0.576 M
How to determine the concentration of sodium ion, Na⁺ ?We'll begin by obtaining the molarity of the solution. This is illustrated below:
Mass of Na₃PO₄ = 23.6 gMolar mass of Na₃PO₄ = 163.94 g/molMole of Na₃PO₄ = 23.6 / 163.94 = 0.144 moleVolume = 750 mL = 750 / 1000 = 0.75 LMolarity = ?Molarity = mole / volume
Molarity = 0.144 / 0.75
Molarity = 0.192 M
Finally we shall determine the concentration of the sodium ion, Na⁺. Details below:
Na₃PO₄(aq) <=> 3Na⁺(aq) + PO₄³⁻(aq)
From the balanced equation above,
1 mole of Na₃PO₄ contains 3 moles of Na⁺
Therefore,
0.192 M Na₃PO₄ will contain = 0.192 × 3 = 0.576 M Na⁺
Thus, the concentration of Na⁺ is 0.576 M
Learn more about molarity:
https://brainly.com/question/13386686
#SPJ1
Acids react with
O water to produce bases and salts
salts to produce bases and water
O neither bases, salts nor water
O bases to produce salts and water
Answers
How to make baking powder
Answers
Answer:
To make baking powder, combine half a teaspoon of cream of tartar and a quarter teaspoon of bicarbonate of soda.
hope it helps;)
Answer:
with baking soda
Explanation:
GIIVNG BRAINLYY!!!! Universal indicator is an acid/base indicator that can be added to a solution to determine its pH. Universal indicator turns solutions different colors based on its pH. A student adds universal indicator to a solution, and the solution turns the color shown below. The solution must be what? view the attachment
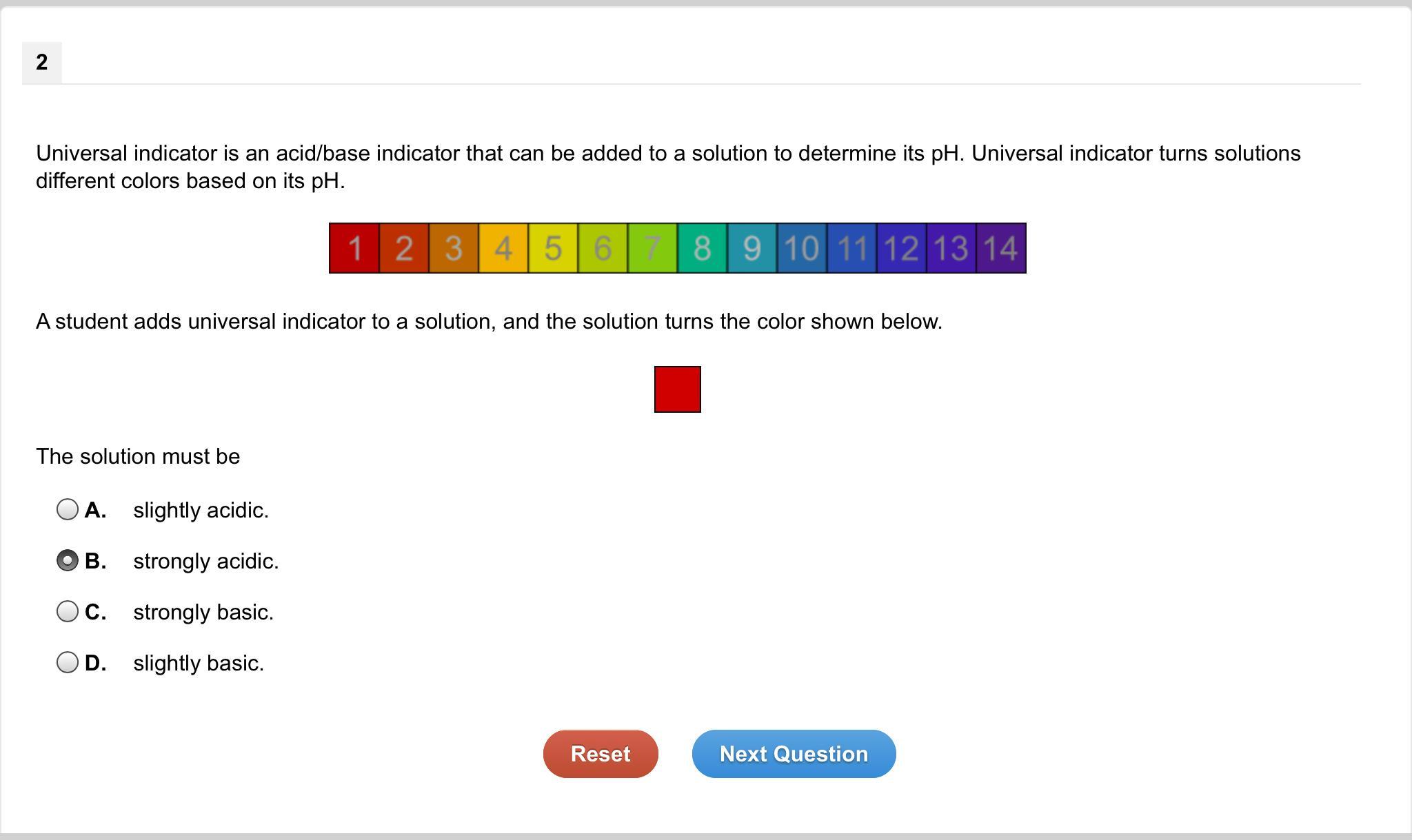
Answers
Answer:
When universal indicator is added to a solution, the color change can indicate the approximate pH of the solution. Acids cause universal indicator solution to change from green toward red. Bases cause universal indicator to change from green toward purple.
Explanation:
The universal indicator turns the solution in red color then the solution must be strongly acidic. Therefore, option (B) is correct.
What is a universal indicator?A universal indicator can be described as a pH indicator made of a solution of various compounds that exhibits many smooth color changes over a wide range of pH values to indicate the acidity or basicity of the solution.
The chemical indicator is a substance that indicates the color change, or the presence/ absence of a threshold amount of a chemical species, such as an acid or base in a solution.
There are many kinds of indicators such as Universal indicator, methyl orange, litmus, phenolphthalein, and bromothymol blue. They are utilized to tell the acidity and basicity of the solution by color change.
In an acidic medium, the universal indicator will show colors such as red, orange, yellow, orangish-yellow, etc. In the basic medium, the universal indicator will change its color to blue-violet or purple. In a neutral medium, it will show green color.
Therefore, the given color is red so the solution is strongly acidic as it has a very low pH value.
Learn more about the universal indicator, here:
https://brainly.com/question/26797119
#SPJ2
How do populations survive when the environment
changes?
Answers
Answer:
adaptation?
Explanation:
Step 1: Determine the total positive charge on the hexapeptide when all acidic and basic groups are fully protonated. Enter your answer without the sign.
Answers
The total positive charge on a hexapeptide when all acidic and basic groups are fully protonated can be calculated by considering the number of positive charges from the basic groups and subtracting the negative charges from the acidic groups.
A hexapeptide is a chain of six amino acids, and its total positive charge depends on the protonation state of the acidic and basic groups present in the peptide. Acidic groups, such as aspartic acid and glutamic acid, can donate protons (H+) and become negatively charged, while basic groups, such as lysine and arginine, can accept protons and become positively charged.
When all acidic and basic groups are fully protonated, this means that all acidic groups have donated their protons and all basic groups have accepted their protons. In this state, all acidic groups will be negatively charged and all basic groups will be positively charged.
To determine the total positive charge on the hexapeptide, we need to calculate the number of positive charges from the basic groups and subtract the negative charges from the acidic groups. The charge on each group depends on its pKa, which is the pH at which half of the group is protonated and half is deprotonated.
The exact pKa values of the basic and acidic groups in a hexapeptide will depend on the specific sequence of amino acids and the chemical environment of the peptide. To accurately determine the total positive charge, the pKa values of the basic and acidic groups in the specific hexapeptide must be known and the pH at which the peptide is being measured must be considered.
To know more about hexapeptide click here:
https://brainly.com/question/17562786#
#SPJ11
Which of the following is a salt that could be generated by combining a weak acid and a weak base? Select the correct answer below: O NaCl Na,SO4 O NH,NO 443 NH F
Answers
The right answer is NH4F.
A salt can be defined as any ionic compound that is composed of positively charged cations and negatively charged anions. A weak acid is an acid that partially dissociates in water to create a relatively little number of hydrogen ions. A weak base is a base that does not completely dissolve in water or only partially ionizes to release hydroxide ions. By reacting a weak acid with a weak base, a salt can be generated.
NH4F is the correct answer because NH4+ is a weak acid and F- is a weak base. When NH4+ is combined with F-, NH4F is formed. NH4F is ammonium fluoride, which is an ionic salt that is made up of ammonium cations (NH4+) and fluoride anions (F-).
To know more about Ammonium Fluoride visit:
https://brainly.com/question/20528587
#SPJ11
How many particles are in 0.75 moles of AgNO3?
Answers
Explanation:
Assume if its asking about molecule particle:
1 mol of AgNO3 = 6.022 x 10^23 molecules
0.75 mol of AgNO3 = 0.75 x 6.022 x 10^23
= 4.5165 x 10^23 molecules
Assume if its asking about atom particle:
AgNO3 has 5 elements
0.75 mol of AgNO3 = 0.75( 5 x 6.022 x 10^23)
= 2.2583 x 10^24 atoms
Put the following in order of most to least. (Hint: convert all to their base unit and then compare.)
20 kilograms
8,776,674,266 micrograms
500 decagrams
1 gigagram
2 teragrams
Answers
The arrangement or order of the mass measurement from the most to the least is 2 teragrams, 1 gigagram, 20 kilograms, 8,776,674,266 micrograms, and the least is 500 decagrams.
What is unit conversion?
Unit conservation is a process in which unit of an object is converted from one form to another.
The unit of mass of the substance is converted as follows;
20 kilograms = 20 kg x 1000 g/kg = 20,000 g
8,776,674,266 micrograms = 8,776,674,266 μg = 8,776,674,266 x 10⁻⁶ g = 8,776.674266 g
500 decagrams = 500 Dg = 500 x 10 = 5,000 g
1 gigagram = 1Gg = 1 x 10⁹ g
2 teragrams = 2 x 10¹² g
The order of the mass of the substance from the most to the least.
2 teragrams1 gigagram20 kilograms8,776,674,266 micrograms500 decagramsThus, the arrangement or order of the mass measurement from the most to the least is 2 teragrams, 1 gigagram, 20 kilograms, 8,776,674,266 micrograms, and the least is 500 decagrams.
Learn more about unit of mass here: https://brainly.com/question/20024683
#SPJ1
Which interaction occurs when light goes into an object as heat energy?
A. Absorbed light
B. Bright light
C. Reflected light
D. Refracted light
Answers
Interaction occurs when light goes into an object as heat energy is Absorbed light.
Absorbed light makes an object dark. Absorption of light is when the light is absorbed by the object and converts it into heat energy. The objects converts the wavelength of light into the larger wavelength of heat. this makes the object warmer and produced energy. Absorption depends on the frequency of light that is transmitted. The wavelength of light defined as the distance between the the two successive troughs of the the light wave.
Therefore, Interaction occurs when light goes into an object as heat energy is Absorbed light.
To learn more about Absorbed light here
https://brainly.com/question/26739493
#SPJ1
suppose you are working with a naoh stock solution but you need a solution with a lower concentration for your experiment. calculate the volume (in ml) of the 1.277 m stock naoh solution needed to prepare 250.0 ml of 0.1236 m dilute naoh solution.
Answers
We need to measure 30.42 ml of the 1.277 M stock NaOH solution and dilute it to a final volume of 250.0 ml to obtain a 0.1236 M dilute NaOH solution.
To prepare 250.0 ml of a 0.1236 M dilute NaOH solution from a 1.277 M stock NaOH solution, we can use the following formula:
C1V1 = C2V2
where C1 is the concentration of the stock solution, V1 is the volume of the stock solution needed, C2 is the concentration of the dilute solution, and V2 is the final volume of the dilute solution.
Substituting the values into the equation, we get:
(1.277 M) V1 = (0.1236 M) (250.0 ml)
V1 = (0.1236 M) (250.0 ml) / (1.277 M)
V1 = 30.42 ml
Therefore, we need to measure 30.42 ml of the 1.277 M stock NaOH solution and dilute it to a final volume of 250.0 ml to obtain a 0.1236 M dilute NaOH solution.
To know more about dilute NaOH solution refer here:
https://brainly.com/question
#SPJ11
Identify the definition that applies to the compound in red. NH3(aq) + H2O(l) → NH4 (aq) + OH-(aq)
a. Arrhenius acid b. Bronsted-Lowry acid c. Arrhenius base d. Bronsted-Lowry base
Answers
The most appropriate definition for NH3 in the given equation is Bronsted-Lowry base, as it accepts a proton from water to form NH4+ and OH-.
The definition that applies to the compound NH3 in the given chemical equation is Bronsted-Lowry base. The Bronsted-Lowry acid-base theory defines an acid as a substance that donates a proton (H+) and a base as a substance that accepts a proton.
In the equation NH3(aq) + H2O(l) → NH4(aq) + OH-(aq), NH3 (ammonia) acts as a base because it accepts a proton (H+) from water, which acts as an acid. The water molecule donates a proton to ammonia, resulting in the formation of the ammonium ion (NH4+) and the hydroxide ion (OH-).
This reaction exemplifies the concept of proton transfer between species in a chemical reaction. According to the Bronsted-Lowry theory, the ammonia molecule (NH3) accepts a proton, making it a base. Meanwhile, water (H2O) donates a proton, making it an acid.
The Arrhenius acid-base theory defines an acid as a substance that releases hydrogen ions (H+) in an aqueous solution, and a base as a substance that releases hydroxide ions (OH-) in an aqueous solution. In the given equation, NH3 does not release H+ ions, so it does not fit the definition of an Arrhenius acid. Similarly, the hydroxide ion (OH-) in the product side does not fit the definition of an Arrhenius base.
Therefore, the most appropriate definition for NH3 in the given equation is Bronsted-Lowry base, as it accepts a proton from water to form NH4+ and OH-.
learn more about Bronsted-Lowry base here
https://brainly.com/question/29317749
#SPJ11
Answer:
D. Bronsted-Lowry base
Explanation:
an unknown solution was determined to be basic. when this solution was combined with lemon juice (a known acid) no visible change occurred. this means that the unknown could be
Answers
The unknown solution could be a variety of basic substances, such as sodium hydroxide, potassium hydroxide, or calcium hydroxide, among others. Further testing and analysis would be needed to determine the exact nature of the unknown solution.
What is Solution?
In chemistry, a solution is a homogeneous mixture composed of two or more substances. The substance present in the largest amount is called the solvent, while the other substances present in smaller amounts are called solutes. The solutes are dissolved in the solvent to form a homogeneous mixture.
When an acid and a base are combined, they can undergo a neutralization reaction that results in the formation of a salt and water. If the acid is added to a basic solution, the resulting mixture will typically have a pH that is closer to neutral than either the acid or the base on their own.
Learn more about Solution
https://brainly.com/question/25326161
#SPJ1
why did plants perform cellular respiration?
Answers
Answer:process of cellular respiration allows plants to break down glucose into ATP. ... Although plants use photosynthesis to produce glucose, they use cellular respiration to release energy from the glucose.
Explanation:
The acid-catalyzed esterification of an alcohol and a carboxylic acid typically has a very favorable equilibrium constant.a. Trueb. False
Answers
The statement that the acid-catalyzed esterification of an alcohol and a carboxylic acid typically has a very favorable equilibrium constant is generally true.
This reaction involves the transfer of a proton from the carboxylic acid to the alcohol, followed by the nucleophilic attack of the alcohol on the carbonyl carbon of the carboxylic acid to form an ester. The acid catalyst speeds up the reaction by promoting the proton transfer step.
In general, esterification reactions are exothermic and produce water as a byproduct, which helps to drive the equilibrium towards the formation of the ester. The equilibrium constant for this reaction can be influenced by factors such as the nature of the alcohol and carboxylic acid, the reaction conditions, and the presence of any catalysts or inhibitors.
However, it should be noted that not all acid-catalyzed esterification reactions will have a favorable equilibrium constant. In some cases, the reaction may be hindered by steric or electronic effects, or by the formation of stable intermediates.
To learn more about; Carboxylic
https://brainly.com/question/28296088
#SPJ11
Upper m g plus upper c a (upper o upper h) subscript 2 right arrow. ? mg(oh)2 + ca no reaction mgh2 + cao mgca + h2o.
Answers
Upper H superscript plus, plus upper O upper H superscript minus right arrow upper H subscript 2 upper O is the equation used to represent the net ionic equation.
What is an ionic reaction?Ionic reactions typically take place while anions and cations interact to form a chemical in a liquid medium. When the ions of saline that are transparent in water interact with anyone, water-insoluble salts are produced.
What benefit does using net ionic equations provide?The benefit of creating an ionic equations is that we're able to identify the ions that are contributing to the reaction as a whole. Since spectator ions can be removed from the electrical equation since they can be from both side of the equation, the net ionic measurement only displays the species that are truly involved in the reaction.
To know more about Ionic equation visit:
https://brainly.com/question/15467502
#SPJ4
Answer: B. no reaction
How many grams of water can be heated from 25°C to 70°C using 15,000 J?
Answers
If a sample of gas is heated from 100◦C to 300◦C at constant pressure, the volume will ____ by a factor of ____
Answers
If a sample of gas is heated from 100◦C to 300◦C at constant pressure. The volume will increase by a factor of approximately 1.82.
If a sample of gas is heated from 100◦C to 300◦C at constant pressure, the volume will increase by a factor of approximately 1.82, assuming that the gas behaves ideally.
This can be calculated using Charles's Law, which states that at constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature.
The absolute temperature can be calculated by adding 273.15 to the Celsius temperature:
V1/T1 = V2/T2
where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature. Converting 100◦C and 300◦C to absolute temperature gives:
V1/T1 = V2/T2
V1/(100 + 273.15) = V2/(300 + 273.15)
V1/373.15 = V2/573.15
V2 = V1 x (573.15/373.15) = V1 x 1.82
For more question on volume click on
https://brainly.com/question/6615047
#SPJ11
A balloon with a volume of 2.0 l at 25°c is placed in a hot room at 35°c. the pressure on the balloon is constant at 1.0 atm. formula to use: v1 t1 = v2 t2 how does the volume of the balloon change after moving it to the hot room?
Answers
The volume increases and the final volume is 2.1 L
Why volume increases?The volume of a gas is directly related to the heat and pressure. If temperature increases, volume increases.
If pressure increases, volume decreases
Based on Boyle's law,
The absolute temperature of a gas is directly proportional to the volume of the gas.
As the balloon change increases its temperature, the volume increases
The Boyle's equation is:
V₁T₂ = V₂T₁
Where,
V is volume T is absolute temperature of 1, initial state and 2, final state of the gas.Replacing :
V₁ = 2.0L
T₂ = 35°C + 273.15 = 308.15K
V₂ = ?
T₁ = 25°C + 273.15 = 298.15 K
2.0L x 308.15 K = V₂ x 298.15 K
2.1 L = V₂
Final volume is 2.1L
Hence, The volume increases and the final volume is 2.1 L
Learn more about gas laws here ;
https://brainly.com/question/12669509
#SPJ1
Answer:
increases, 2.1
Explanation:
I got it right.
I can hit a metal with a hammer without the metal shattering because of its
Malleability
Ductility
Conductivity
Lustrousness
Answers
Answer: Malleability
Explanation: cuz ... its right ... lol
when carbonates react with acids fizzing occurs.what does the fizzing indicate?
Answers
Answer:
acid reacts with carbonates to produce salt, water and CO2. CO2 gas causes bubbling during the reaction, which is observed as fizzing.
Predict whether each of the following oxides is ionic or molecular.
1. Al2O3
2. SnO2
3. CO2
4. H2O
5. Fe2O3
6. Li2O
Answers
1. Al₂O₃ is ionic.
2. SnO₂ is molecular.
3. CO₂ is molecular.
4. H₂O is molecular.
5. Fe₂O₃ is ionic.
6. Li₂O is ionic
The following oxides is ionic or molecular:
1. Al₂O₃ (aluminum oxide) is ionic because it is formed by a metal (Al) and a non-metal (O).
2. SnO₂ (tin oxide) is molecular, as it consists of a metal (Sn) and a non-metal (O).
3. CO₂ (carbon dioxide) is molecular since it is composed of two non-metals (C and O).
4. H₂O (water) is molecular as it is formed by two non-metals (H and O).
5. Fe₂O₃ (iron oxide) is ionic because it contains a metal (Fe) and a non-metal (O).
6. Li₂O (lithium oxide) is ionic as it is composed of a metal (Li) and a non-metal (O).
Learn more about ionic or molecular: https://brainly.com/question/27816573
#SPJ11
Magnesium reacts with sulfuric acid in a single replacement reaction. Which reaction shows the correctly balanced equation?
A. Mg + H₂SO4 →2MgSO4 + H₂
B. 2Mg + H₂SO4 → 2MgSO4 + H₂
C. Mg + H₂SO4 → MgSO4 + H₂
D. Mg + H₂SO4 → H₂MgSO4
Answers
Answer:C. Mg + H₂SO4 → MgSO4 + H₂
Explanation:
A single replacement reaction is a chemical reaction in which an element in a compound is replaced by another element. For example, the reaction between magnesium and sulfuric acid could be represented by the following balanced equation:
Mg + H2SO4 -> MgSO4 + H2
In this equation, the magnesium replaces the hydrogen in the sulfuric acid, forming magnesium sulfate and hydrogen gas.
The correct balanced equation for the reaction between magnesium and sulfuric acid is:
Mg + H2SO4 -> MgSO4 + H2
This equation shows that one molecule of magnesium reacts with one molecule of sulfuric acid to produce one molecule of magnesium sulfate and one molecule of hydrogen gas. The coefficients in front of each compound indicate the number of molecules of each compound that are present in the reaction.
At 20 degrees celcius , the vapor pressur of ethanol is 45 torr and the vapor pressure of methanol is 92 torr. What is the vapor pressure at 20 degrees celcius of a solution prepared by mixing 25 grams of methanol and 75 grams of ethanol ?
Answers
The vapor pressure of a solution prepared by mixing 25 grams of methanol and 75 grams of ethanol at 20 degrees Celsius can be calculated using Raoult's law, which states that the vapor pressure of a component in a solution is directly proportional to its mole fraction.
To calculate the vapor pressure of the solution, we need to use Raoult's law, which is expressed as:
\(P_{total}\)= \(P_A\) × \(x_A\) +\(P_B\) × \(x_B\)
Where \(P_{total}\) is the vapor pressure of the solution, \(P_A\) and \(P_B\) are the vapor pressures of the individual components (methanol and ethanol in this case), and \(x_A\) and \(x_B\) are their respective mole fractions.
First, we need to calculate the mole fractions of methanol ( \(x_A\) ) and ethanol ( \(x_B\)) in the solution. To do this, we need to convert the masses of methanol and ethanol into moles using their molar masses. The molar mass of methanol \((CH_3OH)\) is 32.04 g/mol, and the molar mass of ethanol \((C_2H_5OH)\) is 46.07 g/mol.
Moles of methanol = 25 g / 32.04 g/mol
Moles of ethanol = 75 g / 46.07 g/mol
Next, we calculate the mole fractions:
\(x_A\) = Moles of methanol / (Moles of methanol + Moles of ethanol)
\(x_B\) = Moles of ethanol / (Moles of methanol + Moles of ethanol)
Now that we have the mole fractions, we can substitute them into Raoult's law to calculate the vapor pressure of the solution:
\(P_{total}\) = 92 torr × \(x_A\) + 45 torr × \(x_B\)
Substituting the calculated values of \(x_A\) and \(x_B\) will give us the vapor pressure of the solution at 20 degrees Celsius.
To learn more about Raoult's law refer:
https://brainly.com/question/31597777
#SPJ11
Which kind of weather usually forms over the northwest United States in the summer because of maritime polar air masses? fog dry heat heavy snow heavy rain
HELPPPPP MEEEEEE!!!!!!!!!!!!! T_T
Answers
Answer: SORRY FOR TAKING TOO LONG
Fog is the kind of weather that usually forms over the northwest United States in the summer because of maritime polar air masses. Maritime polar air masses are cool and moist, and when they move over the relatively warmer waters of the Pacific Ocean, they can pick up moisture and become even more humid. When this humid air reaches the cooler air over the land, it can create fog, which is common in the coastal areas of the Pacific Northwest during the summer months.
An element has atomic number 10 and an atomic mass of 20. How many neutrons are in the atom of this element?
OA.
10
OB.
20
O C.
30
D. 0
O E. 200
Answers
10
subtract the atomic mass from the atomic number
1. Which word has the same phoneme as grew, rude, and truth?
A. thumb
B. sure
C. look
D. scoot
Answers
Answer:
B-Sorry if this isn't right :/ Good luck.
Explanation:
Answer:
D. Scoot
Explanation:
Grew, Rude and truth all have the sound /OO/ scoot follows that pattern.