as solution with a ph of 3.40 will have higher concentration of hydroxide ions than hydronium ions true or false
Answers
The statement "As solution with a pH of 3.40 will have higher concentration of hydroxide ions (OH⁻) than hydronium ions (H₃O⁺) " is false.
In acidic solutions, the pH value is less than 7, indicating a higher concentration of hydronium ions compared to hydroxide ions. The pH scale is logarithmic, so each unit decrease in pH represents a tenfold increase in the concentration of hydronium ions.
A pH of 3.40 indicates an acidic solution where the concentration of hydronium ions is higher than the concentration of hydroxide ions. In neutral solutions, the concentration of hydronium ions is equal to the concentration of hydroxide ions, resulting in a pH of 7.
In basic (alkaline) solutions, the concentration of hydroxide ions is higher than the concentration of hydronium ions, resulting in a pH greater than 7.
To know more about the pH refer here :
https://brainly.com/question/2288405#
#SPJ11
Related Questions
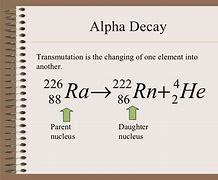
226 • Ra 4/2 He + 88 = ?
Answers
Answer: the answer for this question is in the pic
Explanation:
please some one helpppp!
write the following word equation in to formula equation.
A.sulphur+oxygen=sulfur dioxide B.Aluminum +oxygen=aluminum oxide C.calcium+oxygen=calcium oxide D.sodium+chlorine=sodium chloride E.potassium+oxygen=potassium oxide
Answers
Answer:
A) 2SO2 + O2 = 2SO3
B) Al2O3 + O2 = Al2O3
C) Ca + O2 = CaO2
D) Na (s) + Cl2 (g) = NaCl (s)
E) K + O2 = K2O2
brainliest please
8. Which of the following ground-state electron configurations represents the atom that has the
lowest first-ionization energy?
a) 1s2
b) 1s22s2
c) 1s22s22p6
d) 1s22s22p63s23p1
e) 1s22s22p63s23p3
Answers
Answer:
d) 1s² 2s² 2p⁶ 3s² 3p¹
Explanation:
The lowest ionization energy is found from the predictable nature of ionization energy knowing that we have for ionization energy;
1) The ionization energy becomes progressively larger as we move from left to right along the periodic table
2) The ionization energy becomes progressively larger or increases as we move from the top to the bottom in a particular group within the periodic table
Therefore, atoms which are far right (have more electrons in their valence shells) have higher ionization energies, while the atoms that are at the bottom of a group (with more shells in their electronic configuration) have lower ionization energy which gives;
1s² = Helium
1s² 2s² = Beryllium
1s² 2s² sp⁶ = Neon
1s² 2s² 2p⁶ 3s² 3p¹ = Aluminum
1s² 2s² 2p⁶ 3s² 3p³ = Phosphorus
The ionization energy of 1s² 2s² 2p⁶ has the highest ionization energy, while the atom 1s² 2s² 2p⁶ 3s² 3p¹ which has one electron in the p orbital, has the lowest ionization energy
1s² 2s² 2p⁶ 3s² 3p¹ which is Aluminum represents the atom with the lowest
first-ionization energy
Ionization energy is the energy that is needed to remove an electron from its orbital during chemical reactions.
The atoms with more electrons in their outer shells have higher ionization
energies, while the atoms with lesser electrons in their outer shells have
lower ionization energy.
1s²
1s² 2s²
1s² 2s² 2p⁶
1s² 2s² 2p⁶ 3s² 3p¹
1s² 2s² 2p⁶ 3s² 3p³
The element with the lowest first-ionization energy is therefore 1s² 2s² 2p⁶
3s² 3p¹ and the element with the highest first-ionization energy will be 1s²
2s² 2p⁶ .
Read more on https://brainly.com/question/17783060
what is Very small and located on ER or located
floating on its own in the cytoplasm, it is
responsible for making proteins for repair and
growth.
Answers
do stearic acid molecules exists as rectangular prisms
Answers
No, stearic acid molecules do not exist as rectangular prisms.
Stearic acid, also known as octadecanoic acid, is a saturated fatty acid that exists in the form of a chain of 18 carbon atoms with a carboxyl group (-COOH) at one end. The molecule has a linear structure and is not shaped like a rectangular prism.
In fact, no molecules exist as rectangular prisms. Molecules are made up of atoms bonded together, and the shape of a molecule is determined by the arrangement of these atoms and the types of bonds between them. Most molecules have complex, three-dimensional shapes that are not easily described as simple geometric shapes like rectangular prisms.
To know more about stearic acid click here:
https://brainly.com/question/13200535#
#SPJ11
Perform the following
mathematical operation, and
report the answer to the
appropriate number of
significant figures.
1204.2 +4.79613 = [ ? ]

Answers
This problem is providing a mathematical expression which the result should be expressed with the correct significant figures. At the end, the result is 1209.0 because of the following:
Significant figures:In science, the use of significant figures is crucial as long numbers are not necessarily required when reporting a numerical value, for that reason the importance of reporting measurements with the correct number of significant figures.
In the case of additions, we perform the normal operation as the first step:
1204.2 +4.79613 = 1208.99613
Next, we round the result to the least number of decimal places, in this case one because 1204.2 has just one decimal place, unlike the 4.79613 which has five, so that we round the 8 up to 9 and leave a 0 as the only decimal place:
1209.0
Learn more about significant figures: https://brainly.com/question/11904364
Given the pk, of each acid, determine whether it is strong or weak. a. citric acid, pka=3.1 _________ b. acetic acid, pka=4.7 _________c. sulfuric acid, pKq=-5 __________ d. nitric acid, pkg=-2 __________-
Answers
From the pK we can determine that citric acid is a weak acid, acetic acid is weak acid, sulfuric acid is a strong acid, nitric acid is a strong acid.
In layman's terms, pKa is a measurement of an acid's strength. A strong acid will have a pKa value that is lower than 0. To be more specific, pKa is the Ka value's negative log base ten value (acid dissociation constant). How tightly a proton is retained by a Bronsted acid is how the strength of an acid is measured. The strength of the acid and its capacity to donate protons increase with decreasing pKa values.
Therefore, Citric acid is a weak acid, acetic acid is a weak acid, sulfuric acid is a strong acid, and nitric acid is a strong acid, according to the pK.
learn more about strong and weak acid at https://brainly.com/question/12811944
#SPJ4
which best explains why water has a much higher boiling point than would otherwise be predicted?water forms weak hydrogen bonds.
Answers
Water has a higher boiling point than would be expected due to the formation of weak hydrogen bonds. These bonds occur between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of neighboring water molecules.
The hydrogen bonds create a network of intermolecular forces that hold the water molecules together, requiring more energy to break them apart and transition to a gaseous state.
The strength of these hydrogen bonds also explains why water has a high surface tension and is able to dissolve a wide range of substances.
The unique properties of water are essential for supporting life on Earth and have important implications for various industries such as chemistry and engineering.
Understanding the chemistry behind these properties can help us better appreciate the role of water in our daily lives.
To Learn more about hydrogen Click this!
brainly.com/question/6774224
#SPJ11
Be as explicit as you can in describing how the covalent bond between an atom of Chlorine and an atom of Iodine forms. (What happens to the electrons, which electrons are involved, and what allows this to happen MUST all be part of your answer to receive full credit.)
Answers
When two atoms of chlorine and iodine join together to form a covalent bond, their outermost electrons interact to form a chemical bond.
What is a Covalent bond?
A covalent bond is a type of chemical bond that involves the sharing of a pair of electrons between two atoms. Covalent bonds are formed when two atoms come together to share their electrons in order to reach a more stable electron configuration. As a result, the two atoms become bonded together by the attraction of their shared electrons.
The chlorine atom has seven valence electrons, while the iodine atom has seven as well. Both atoms will want to complete their octets, so the two atoms share their outermost electrons with each other. During the formation of the covalent bond, the chlorine atom will donate one of its electrons to the iodine atom, while the iodine atom will donate one of its electrons to the chlorine atom. This sharing of electrons allows the two atoms to form a single bond and fill their octets, forming a covalent bond.
To know more about a Covalent bond,
https://brainly.com/question/3447218
#SPJ4
No underwater luminaires shall be installed in a permanently-installed swimming pool that operates on supply circuits over ? between conductors.
Answers
No luminaires shall be installed for operation on supply circuits over 150 volts between conductors. Luminaires mounted in walls shall be installed with the top of the luminaire lens not less than 450 mm (18 in.)
In an electrical installation, what is a luminaire?
A luminaire, also known as a light fixture, is a complete lighting assembly that includes one or more lamps (light bulbs or tubes), a socket and other protective elements, wiring that links the lamp to a power source, and a reflector to help direct and spread the light.
The construction, installation, or inclusion of shades or guards on luminaires is required to prevent the exposure of combustible materials to temperatures above 90°C (194°F). Unswitched lamp holders must be used above materials that are very flammable.
Learn more about luminaire from
brainly.com/question/4333610
#SPJ4
Help pls what is the (H+) of a solution with a pH of 4?
a. 1E-4 M
b. 0.602 M
c. 1E6 M
d. 1E4 M
Answers
Answer:
A
Explanation:
[H+] = 10^-pH
[H+] = 1 x 10^-4 M
Answer :A
What process transfers water from the atmosphere to the hydrosphere
A evaporation
B runoff
C precipitation
D currents
Answers
1. calculate the ph of a buffer solution made from equal amounts of 0.30 m hydrofluoric acid and 0.70 m sodium fluoride. ka=7.1×10−4
Answers
A buffer solution can resist a change in pH even when a strong acid or a strong base is added to it. A buffer solution is made up of a weak acid and its conjugate base, or a weak base and its conjugate acid.A hydrofluoric acid-sodium fluoride buffer solution can be made from hydrofluoric acid and sodium fluoride.
The buffer solution can be calculated as follows: Hydrofluoric acid is a weak acid, with a Ka of 7.1 × 10−4.Moles of Hydrofluoric acid (HF) = 0.30 × VolumefHF = [HF]/V = 0.30 mMoles of sodium fluoride (NaF) = 0.70 × VolumefNaF = [NaF]/V = 0.70 mMoles of Hydrogen Fluoride (H+) = Molarity × Volume = 0.30 × VolumepH = pKa + log ([A-]/[HA])Ka = [H+][A-]/[HA]7.1 × 10−4 = [H+][NaF]/[HF][H+] = 5.3 × 10−4[Naf]/[HF] = 7/3log [NaF]/[HF] = log (7/3) = 0.851pH = pKa + log ([A-]/[HA])pH = 3.86 + 0.851 = 4.71Therefore, the pH of a buffer solution made from equal amounts of 0.30 M hydrofluoric acid and 0.70 M sodium fluoride is 4.71.
For more information on buffer solution visit:
brainly.com/question/31428923
#SPJ11
37 grams of H3PO4 ????
Answers
Answer:
76 grams is the correct answer to this question
Which statement about atoms is false ?

Answers
Answer:
Following Statement is false: Atoms have an overall negative charge
Explanation:
hope it helps
Which element is placed in the same period as ruthenium but has a higher atomic number than it?
A.bismuth
B.osmium
C.silver
D.zirconium
Answers
Answer:
The part that is put in the same time as ruthenium, but has a greater number of atoms than silver.
Answer:
The answer is silver.
Explanation:
edge 2022, trust
when does the digestion happen?
Answers
Answer:
The processes of digestion include five activities: Ingestion, Digestion, absorption, assimilation and egestion.
Ingestion- Ingestion is the consumption of a substance by an organism. In animals, it normally is accomplished by taking in a substance through the mouth into the gastrointestinal tract, such as through eating or drinking. In single-celled organisms ingestion takes place by absorbing a substance through the cell membrane.
Digestion- Digestion is the complex process of turning the food you eat into nutrients, which the body uses for energy, growth and cell repair needed to survive. The digestion process also involves creating waste to be eliminated.
Absorption- The simple molecules that result from chemical digestion pass through cell membranes of the lining in the small intestine into the blood or lymph capillaries. This process is called absorption.
Assimilation- Assimilation is the movement of digested food molecules into the cells of the body where they are used. For example: amino acids are used to build new proteins.
Egestion- Egestion is the act of excreting unusable or undigested material from a cell, as in the case of single-celled organisms, or from the digestive tract of multicellular animals.
Which solid below is held together by all mobile electrons being shared?
1) CS₂ 2)NaCl
3)Ni 4)Br₂
Answers
the diels-alder mechanism between a diene and a dienophile is choose... , which means that bond breaking happens choose... bond forming. to help the mechanism succeed, the diene should have an choose... group and the dienophile should have an choose... group.
Answers
The Diels-Alder mechanism among a diene and a dienophile is a concerted reaction, because of this that bond breaking occurs simultaneously with bond forming.
To help the mechanism be triumphant, the diene have to have an electron liberating organization and the dienophile have to have an electron taking flight organization.
A concerted reaction is a form of chemical response where all of the bond-forming and bond-breaking steps arise concurrently, in a single step. In other phrases, all of the reactants come collectively and react to shape the products with none intermediate steps.
Concerted reactions also are referred to as pericyclic reactions due to the fact they contain the formation and breaking of bonds in cyclic transition states. these reactions comply with a specific set of rules referred to as the Woodward-Hoffmann guidelines, which describe the allowed and forbidden modes of overlap among the orbitals of the reactants and products. These reactions are often utilized in organic synthesis to create complicated molecules with excessive stereoselectivity and regioselectivity.
To learn more about Concerted reaction visit here:
brainly.com/question/29584139
#SPJ4
Complete Question:
The Diels-Alder mechanism between a diene and a dienophile is _____, which means that bond breaking happens _____ as bond forming.
To help the mechanism succeed, the diene should have an _____ group and the dienophile should have an ______ group.
The standard Entropy and Enthalpies for four (4) reactions, A-D, are presented. Reaction ΔΗο (k/mol) So (J/mol K) -72.0 61.0 58.0 68.0 19.0 30.0 -40.0 33.0 sing this data, order these reactions from least produ't favored to most product favored under standard conditions:
Answers
Order of reactions from least product-favored to most product-favored under standard conditions: D < A < B < C.
The order is determined based on the Gibbs free energy change (ΔG) at standard conditions, using the equation ΔG = ΔH - TΔS, where T is the temperature (usually 298 K). A more negative value of ΔG indicates a more product-favored reaction.
By calculating ΔG for each reaction, we find that reaction D has the highest ΔG, indicating it is the least product-favored. Reaction A has a slightly lower ΔG than D but is still less product-favored. Reaction B has a more negative ΔG than A, indicating a higher degree of product formation. Finally, reaction C has the most negative ΔG, suggesting it is the most product-favored among the four reactions under standard conditions.
Please note that this ordering assumes the reactions are reversible and that the given values are for the forward reaction.
learn more about reaction here:
https://brainly.com/question/30464598
#SPJ11
Q2.
A student uses this apparatus to find the temperature change when sodium hydroxide solution
reacts with dilute hydrochloric acid.
polystyrene cup
1
This is the student's method.
• pour 20 cm of sodium hydroxide solution into a polystyrene cup
• record the temperature of the sodium hydroxide solution
• add 20 cm of dilute hydrochloric acid and stir the mixture
• record the highest temperature of the mixture
(a) () Give the formula of the ion that causes sodium hydroxide solution to be alkaline.
(1)
(1) Give the formula of the ion that causes hydrochloric acid to be acidic.
(1)
(1) Write the net ionic equation for neutralization reaction of sodium hydroxide with
hydrochloric acid
(1)
(iv) Suggest a pH value for the dilute hydrochloric acid.
(1)
1193 words
X
English (United States)
Focus
979
Answers
g the remainder of the acids are called weak acids. how do weak acids differ from strong acids in solution? explain using the ph and conductivity behavior observed.
Answers
A strong acid is one that entirely ionizes in aqueous solution. When dissolved in water, it always loses a proton (H+). Acid that only partially ionizes in a solution is considered weak acid.
Only a small number of its (H+) atoms are released when it dissolves in water and it also can be explained by Conductivity .
What is Conductivity ?An electrolyte solution's conductivity is a gauge of how well it conducts electricity. In the SI, conductivity is measured in Siemens per meter.
Conductivity is a helpful indicator of water quality in general. Every body of water has a typical conductivity range that, once determined, can be used as a reference point for comparison with routine conductivity tests.
While electrical conductance is a characteristic of a specific electrical component, electrical conductivity is a quality of the substance itself (such as silver) (like a particular wire). The amount of voltage needed to for a given amount of electric current to flow is known as electrical conductivity.
What are weak acids ?An acid that partially separates into its ions in water or an aqueous solution is referred to as a weak acid. On the other hand, in water, a strong acid completely dissociates into its ions. While the conjugate acid of a weak base is also a weak acid, the conjugate base of a weak acid is also a weak base.
The ability of acidic and basic solutions to conduct electricity is referred to as strong and weak. Strong electrical conductivity indicates that an acid or base is a strong acid or base. A weak acid or base is one that conducts electricity only slightly.
To know more about Conductivity please click here ; https://brainly.com/question/24943129
#SPJ4
In chemical communication between cells, a ________ cell secretes a chemical messenger that binds to ________ on the ________ cell.
Answers
In chemical communication between cells, a secretory cell secretes a chemical messenger that binds to receptors on the target cell.
Cells are the basic building blocks of all living things. The human body is made up of trillions of cells. They give structure to the body, absorb nutrients from food, convert those nutrients into energy, and perform specific functions. In multicellular organisms, such as higher plants and animals, specialized cell groups are organized into tissues and organs.
Cells can be used as the basis for describing organisms as unicellular or multicellular. Unicellular organisms are organisms that have only one cell, i.e. single-celled organisms. Prokaryotes and protists are examples. Multicellular organisms are organisms with more than one cell. Examples are plants and animals.
Learn more about cell here
https://brainly.com/question/13920046
#SPJ4
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG°for the following redox reaction. Round your answer to 4 significant digits. 2H20 (1)+4Cu²+ (aq) 02(g) +4H+ (aq) +4Cu (aq) x | ?
Answers
The standard reaction free energy AG° for the given redox reaction is \(1.320 x 10^5 J/mol.\)
Calculate AG° for 2H20 + 4Cu²+ → 02 + 4H+ + 4CuThe balanced equation for the given redox reaction is:
2H₂(l) + 4Cu₂+(aq) + O₂(g) + 4H+(aq) → 4Cu(s) + 4H₂O(l)
The half-reactions involved in this reaction are:
O₂(g) + 4H+(aq) + 4e- → 2H₂O(l) E° = +1.23 V
Cu₂+(aq) + 2e- → Cu(s) E° = +0.34 V
To determine the standard reaction free energy AG°, we can use the following equation:
AG° = -nFE°
where:
n is the number of electrons transferred in the reaction (in this case, n = 4)
F is the Faraday constant (96,485 C/mol)
E° is the standard cell potential, which can be calculated as the difference between the reduction potential of the cathode and the anode (E°cathode - E°anode)
Using the given standard reduction potentials, we have:
E°cell = E°cathode - E°anode
E°cell = (+0.00 V) - (+0.34 V) = -0.34 V
Since the reaction involves the transfer of 4 electrons, we have:
AG° = -nFE°
AG° = -(4 mol e-)(96,485 C/mol)(-0.34 V)
AG° = 131,973 J/mol
Rounding this to 4 significant digits gives:
AG° = \(1.320 x 10^5\)J/mol
Learn more about reaction
brainly.com/question/17434463
#SPJ11
what is the balanced equation when aluminum reacts with copper(ii) sulfate
Answers
When aluminum reacts with copper (II) sulfate, the balanced equation is given as follows:2Al(s) + 3CuSO₄(aq) → Al₂(SO₄)₃(aq) + 3Cu(s).
The balanced equation when aluminum reacts with copper (II) sulfate can be obtained by following these steps;
Step 1: Write the unbalanced equation . Aluminum and copper (II) sulfate react to give aluminum sulfate and copper.
The equation is given as; Al(s) + CuSO₄(aq) → Al₂(SO₄)₃(aq) + Cu(s)
Step 2: Balance the equation- The unbalanced equation above shows that there are two aluminum atoms on the left-hand side (LHS) of the equation and two aluminum atoms on the right-hand side (RHS) of the equation.To balance the equation, we have to make sure that the number of atoms of each element on the LHS of the equation is equal to the number of atoms of the same element on the RHS of the equation.
We can balance the equation by following these steps;
Add a 3 in front of Al₂(SO₄)₃(aq) to balance the aluminum atoms as follows:
Al(s) + CuSO₄(aq) → 3Al₂(SO₄)₃(aq) + Cu(s)
Now, we have 6 aluminum atoms on the LHS of the equation and 6 aluminum atoms on the RHS of the equation.
Add a 2 in front of CuSO₄(aq) to balance the copper and sulfur atoms as follows:
Al(s) + 2CuSO₄(aq) → 3Al₂(SO₄)₃(aq) + 2Cu(s)
Now, we have 2 copper atoms and 8 sulfur atoms on both sides of the equation.
Therefore, the balanced equation when aluminum reacts with copper (II) sulfate is:
2Al(s) + 3CuSO₄(aq) → Al₂(SO₄)₃(aq) + 3Cu(s).
When aluminum reacts with copper (II) sulfate, the balanced equation is given as follows:2Al(s) + 3CuSO₄(aq) → Al₂(SO₄)₃(aq) + 3Cu(s).
Learn more about balanced equation
brainly.com/question/7181548
#SPJ11
A 748 L hot air balloon sits on a table at 26.7oC. If the balloon is heated to 48.3oC, what is the new volume?
Answers
The new volume of the hot air balloon is 801.9L.
As the temperature of the hot air balloon on the table is increased, the its volume also increases.
Charle's lawCharle's law states that the volume of an ideal gas is directly proportional to the absolute temperature provided pressure is kept at constant.
It is expressed as;
V₁/T₁ = V₂/T₂
Given the data in the question;
Initial volume V₁ = 748LInitial temperature T₁ = 26.7°C = 299.85KFinal temperature T₂ = 48.3°C = 321.45KFinal volume V₂ = ?To calculate the new volume we subtsitute our given values into the expression above.
V₁T₂ = V₂T₁
V₂ = V₁T₂ / T₁
V₂ = ( 748L × 321.45K ) / 299.85K
V₂ = 240444.6LK / 299.85K
V₂ = 801.9L
The new volume of the hot air balloon is 801.9L.
As the temperature of the hot air balloon on the table is increased, the its volume also increases.
Learn more about Charle's law here: https://brainly.com/question/21184611
a city council is debating between two potential water purification systems: reverse osmosis and ion exchange. cost is the primary criteria for the choice. which decision is the most likely result of this debate?(1 point)
Answers
It is likely that they would choose the ion exchange system as the most cost-effective option for water purification, provided it meets their specific water quality requirements.
Based on the student question, it appears that the city council is considering two water purification systems, reverse osmosis and ion exchange, with cost being the primary criterion for their decision.
In this scenario, the most likely result of the debate would be the selection of the water purification system with the lowest overall cost, taking into account both initial investment and ongoing operational expenses. To determine this, the city council would need to conduct a thorough cost analysis of each system.
Reverse osmosis is a process that uses pressure to force water through a semi-permeable membrane, removing contaminants and impurities. It is an effective method for purifying water, but the process can be energy-intensive and may require significant infrastructure investments, such as high-pressure pumps and specialized membranes. Additionally, ongoing costs can be high due to membrane replacement and energy usage.
for more such questions on water quality :
https://brainly.com/question/23717398
#SPJ11
how to separate water from aqueous magnesium sulphate?
Answers
Answer:
The method generally involves heating a mixture of epsomite and halite to form clusters of lower hydrated magnesium sulfate crystals and subsequently applying slight pressure to the clusters so that they collapse to yield fine, less hydrated magnesium sulfate crystals which can then be easily separated from the ...
We have that This Separation is done by heating the Mixture to form magnesium sulfate crystals
Pressure is then applied to the magnesium sulfate crystals causing a collapse to give Reduced hydration of magnesium sulfate crystals
From the question we are told
How to separate water from aqueous magnesium sulphate?
Generally
Water
the is a composition of hydrogen and Oxygen at a required Proportion and it exists in gaseous state
aqueous magnesium sulfate
the is a composition of magnesium and Sulfur at a required Proportion and exists in aqueous
Therefore
This Separation is done by heating the Mixture to form magnesium sulfate crystals
Pressure is then applied to the magnesium sulfate crystals causing a collapse to give Reduced hydration of magnesium sulfate crystals.
For more information on this visit
https://brainly.com/question/1641336
The pressure P (in kilopascals), volume V (in liters), and temperature T (in kelvins) of a mole of an ideal gas are related by the equation PV=8.31T. Find the rate at which the volume is changing when the temperature is 295 K and increasing at a rate of 0.05 K/s and the pressure is 16 and increasing at a rate of 0.02kPa/s. Please show your answers to at least 4 decimal places.
dV/dt =
Answers
The rate at which the volume is changing, represented as dV/dt, is given by the equation (0.4155 - 0.32V(t)) / 16, where V(t) is the volume, and the values are substituted accordingly.
To find the rate at which the volume is changing, we need to differentiate the given equation with respect to time (t) using the chain rule:
PV = 8.31T
Differentiating both sides with respect to time:
P(dV/dt) + V(dP/dt) = 8.31(dT/dt)
We are given:
dT/dt = 0.05 K/s (rate of temperature change)
(dP/dt) = 0.02 kPa/s (rate of pressure change)
P = 16 kPa (initial pressure)
T = 295 K (initial temperature)
Substituting the given values into the equation, we have:
16(dV/dt) + 16V(0.02) = 8.31(0.05)
Simplifying the equation:
16(dV/dt) + 0.32V = 0.4155
Rearranging the equation to solve for dV/dt:
16(dV/dt) = 0.4155 - 0.32V
(dV/dt) = (0.4155 - 0.32V) / 16
To find the rate at which the volume is changing when T = 295 K, we substitute V = V(t) and T = 295 into the equation:
(dV/dt) = (0.4155 - 0.32V(t)) / 16
Calculating the value of (dV/dt) at the given temperature and rounding to at least 4 decimal places will provide the final answer.
learn more about temperature change here:
https://brainly.com/question/31788620
#SPJ11
What would be the bond angle if the molecular geometry were bent and had only one lone pair of electrons
Answers
120°
Bent molecular geometry
Examples H2O, SO2
Point group C2v
Coordination number 2
Bond angle(s) 90°<θ<120°