Answers
The ionization energy grows from left to right during the course of a period and drops from top to bottom in groups. As a result, helium has the highest initial ionization energy whereas francium has one of the lowest.
Why is ionization necessary?We utilize ionizing radiation on a daily basis to keep ourselves healthy. Smoke detectors emit ionizing radiation, which is also employed in many other aspects of daily life, including the sterilization of blood and medical equipment. It is a byproduct of the nuclear power industry as well.
What kind of energy is ionization?The amount of energy required for an isolated, gaseous atom to discharge one electron, creating a cation, is known as the ionization energy. Typically, kJ/mol is used to express this energy.
To know more about Ionization visit:
https://brainly.com/question/28385102
#SPJ4
Related Questions
HELP! URGENT Which of the following best states the relationship between erosion and deposition?
A.
When the energy transporting sediments diminishes, the sediments settle in a low-lying area; therefore, deposition always follows erosion.
B.
When the energy transporting sediments diminishes, the sediments settle in a low-lying area; therefore, erosion always follows deposition.
C.
When rock is broken down into sediments, the sediments are eventually transported to another location; therefore, deposition is a form of erosion.
D.
When rock is broken down into sediments, the sediments are eventually transported to another location; therefore, erosion is a form of deposition.
Answers
Which refers to the passing of a wave through an object?
sound
O interference
O transmission
O frequency
O sound
Answers
The term that refers to the passing of a wave through an object is "transmission."
Transmission refers to the process by which a wave passes through an object or medium. In the context of sound, transmission occurs when sound waves travel through different substances, such as air, water, or solids.
When a sound wave encounters an object, it can be transmitted through it, reflected off it, or absorbed by it, depending on the properties of the object and the medium through which the sound is traveling.
For example, when you speak into a microphone, the sound waves produced by your voice travel through the air and are transmitted to the microphone's diaphragm. The diaphragm converts the sound waves into electrical signals, which can then be amplified and reproduced as sound through speakers.
In summary, transmission is the term used to describe the passage of a wave, such as a sound wave, through an object or medium. It is an essential concept in understanding how waves interact with their surroundings and how sound propagates through different materials.
for such more questions on transmission
https://brainly.com/question/18451537
#SPJ8
When 6.0 mol Al react with 13 mol HCl what is the limiting reactant and how many moles can be formed
2Al + 6HCl - 2AlCl3 3H2
Answers
The limiting reactant in the reaction is HCl
The amount of products that can be formed in the reaction is:
4.33 moles of AlCl₃
6.5 moles of H₂
What is a limiting reactant?A limiting reactant is a reactant that is used up in a reaction.
The limiting reactant when once used up, and the reaction stops. Thus, the limiting reactant produces the least amount of product from the reactants that are present.
Considering the given reaction:
2 Al + 6 HCl ---> 2 AlCl₃ + 3H₂
The mole ratio of the reactants aluminum and hydrochloric acid is 2 : 6
Hence, 13 moles of HCl will require 13 * 2/6 moles of Al = 4.33 moles of Al
Hence, HCl is the limiting reactant.
The amount of products that can be formed is:
AlCl₃ = 13 * 2/6
AlCl₃ = 4.33 moles
H₂ = 13 * 3/6
H₂= 6.5 moles
Learn more about limiting reactants at: https://brainly.com/question/19033878
#SPJ1
When Sam plays online video games with other people he makes sure to avoid offensive language play by the rules his gaming group established and help other players have a good time. Sam is being a good digital citizen by following a code of
Answers
"Sam is being a good digital citizen by following a code of conduct.
A code of conduct is a set of rules that govern how people should behave online. It is important to follow a code of conduct because it helps to create a safe and respectful environment for everyone.
Here, Sam is following the code of conduct for his gaming group. This code of conduct may include rules about using offensive language, cheating, and griefing. By following these rules, Sam is helping to create a positive experience for everyone in his gaming group.
Therefore, Sam is following the code of conduct.
Learn more on code of conduct :https://brainly.com/question/30093328
#SPJ1
8. There are 2850.5 miles between Houston, TX and Vancouver, Canada, site of the 2010 Olympic Games. How many
meters is that equal to if 1 mile is equal to 1.6 km? Express your answer in scientific notation
2850.5
9. A newborn baby eats & times a day At.

Answers
Answer:
4560.8km
Explanation:
2,850.5×1.6 = 4560.8km
How many moles of silver chloride will be produced if 2 moles of silver is allowed to react with an unlimited amount of chlorine?
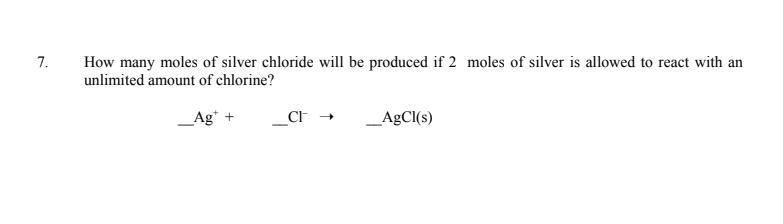
Answers
If 2 moles of silver is allowed to react with an unlimited amount of chlorine, then 4 moles of silver chloride will be produced. This is because the reaction between silver and chlorine follows the following equation:
2Ag + Cl2 → 2AgCl
Therefore, for every 2 moles of silver, 2 moles of silver chloride will be produced, so 4 moles of silver chloride will be produced if 2 moles of silver is allowed to react with an unlimited amount of chlorine.
What could be done to improve how plants are categorized?
Answers
Answer:
While there are many ways to structure plant classification, one way is to group them into vascular and non-vascular plants, seed bearing and spore bearing, and angiosperms and gymnosperms.
Explanation:
What is the number of molecules of 140 g of CO2?
Answers
Answer:
We have been given a quantity of carbon monoxide, CO, which is 140 g. we have to find the number of molecules in this quantity of carbon monoxide. Hence, 140 g of CO has 3.02×1024molecules.
Explanation:
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
How many chiral centers does clavulanic acid have?
Answers
Answer:
Clavulanic acid has two (2) chiral centers.
Explanation:
A chiral center is a center (usually carbon) with four different substituents.
The structure of clavulanic acid is shown in the attachment below.
Consider the labeled diagram in the attachment,
Carbon A is not a chiral carbon because it has two hydrogen atoms attached to it
Carbon B is not a chiral carbon because it has only three substituents
Carbon C is a chiral carbon because it has four different substituents
Carbon D is a chiral carbon because it has four different substituents
Carbon E is not a chiral carbon because it has only three atoms directly attached to it
Carbon F is not a chiral carbon because it has only three atoms directly attached to it
Carbon G is not a chiral carbon because it has two hydrogen atoms attached to it
Carbon H is not a chiral carbon because it has only three substituents
Then, only carbons C and D are chiral carbons.
Hence, clavulanic acid have two (2) chiral centers.

calculate the pH of the solution obtained if 40cm^3 of 0.2M HCl was added to 30cm^3 of 0.1M NaOH
Answers
To calculate the pH of the solution obtained by mixing HCl and NaOH, we need to consider the neutralization reaction between the two compounds. The reaction between HCl (hydrochloric acid) and NaOH (sodium hydroxide) produces water (H₂O) and forms a salt (NaCl).
Given:
Volume of HCl solution (V₁) = 40 cm³
Concentration of HCl solution (C₁) = 0.2 M
Volume of NaOH solution (V₂) = 30 cm³
Concentration of NaOH solution (C₂) = 0.1 M
1. Determine the moles of HCl and NaOH used:
Moles of HCl = Concentration (C₁) × Volume (V₁)
Moles of HCl = 0.2 M × 0.04 L (converting cm³ to L)
Moles of HCl = 0.008 mol
Moles of NaOH = Concentration (C₂) × Volume (V₂)
Moles of NaOH = 0.1 M × 0.03 L (converting cm³ to L)
Moles of NaOH = 0.003 mol
2. Determine the limiting reagent:
The stoichiometry of the reaction between HCl and NaOH is 1:1, meaning that they react in a 1:1 ratio. Whichever reactant is present in a smaller amount will be the limiting reagent.
In this case, NaOH is present in a smaller amount (0.003 mol), which means it will be fully consumed during the reaction.
3. Determine the excess reagent and its remaining moles:
Since NaOH is the limiting reagent, we need to find the remaining moles of HCl.
Moles of HCl remaining = Moles of HCl initially - Moles of NaOH reacted
Moles of HCl remaining = 0.008 mol - 0.003 mol
Moles of HCl remaining = 0.005 mol
4. Calculate the concentration of HCl in the resulting solution:
Volume of resulting solution = Volume of HCl solution + Volume of NaOH solution
Volume of resulting solution = 0.04 L + 0.03 L
Volume of resulting solution = 0.07 L
Concentration of HCl in the resulting solution = Moles of HCl remaining / Volume of resulting solution
Concentration of HCl in the resulting solution = 0.005 mol / 0.07 L
Concentration of HCl in the resulting solution ≈ 0.071 M
5. Calculate the pH of the resulting solution:
pH = -log[H⁺]
pH = -log(0.071)
Using logarithm properties, we can determine the pH value:
pH ≈ -log(0.071)
pH ≈ -(-1.147)
pH ≈ 1.147
Therefore, the pH of the solution obtained by mixing 40 cm³ of 0.2 M HCl and 30 cm³ of 0.1 M NaOH is approximately 1.147.
the mass of a single potassium atom is 6.50×10-23 grams. How many potassium atoms would there be in 114 milligrams of potassium?
Answers
Answer:LOL
42
Explanation:
The mass of a single potassium atom is 6.50×10⁻²³ grams potassium atoms would be in 114 milligrams of potassium 17.53 ×10⁻²³.
What is an atom?
An atom is the most diminutive form of any chemical compound that takes part in the chemical reaction to form any product and it is equal to the one mole which is 2.303 ×10²³ moles of the Avogadros number.
To calculate the number of atoms we have, 6.50×10⁻²³ grams of potassium and the value of one particle is 2.303 ×10²³ moles,
atoms = 6.50×10⁻²³ / 114 milligrams of potassium
atoms of potassium = 17.53 ×10⁻²³. atoms.
Therefore, 17.53 ×10⁻²³.atoms are present if the mass of a single potassium atom is 6.50×10⁻²³ grams potassium atoms would there be in 114 milligrams of potassium.
Learn more about atoms, here:
https://brainly.com/question/15488332
#SPJ2
Compute for the pH of 0.415 M HCl acid.
Answers
Answer:
The answer is
0.38Explanation:
HCL is a strong acid and therefore undergo complete dissociation
We have
\( HCL \rarr H^{ + } \: + \: Cl^{ - } \)The concentration of Hydrogen ion is also 0.415 M
The pH of a solution can be found by using the formula
\(pH = - log( {H }^{ + } ) \)
So we have
\(pH = - log(0.415) \\ = 0.381951903...\)
We have the final answer as
0.38Hope this helps you
a wooden baseball bat is an example of
a: compound
b: element
c: heterogeneous
d: homogeneous
Answers
Compound or element
A wooden baseball bat is an example of a heterogeneous substance.
A heterogeneous substance is consists of compounds that are not thoroughly mixed together.
Woods are fibrous structural substances with spaces present in trees and other woody plants' stems as well as roots. They are composed of a natural composite of cellulose fibers and are contained with a lignin matrix that is capable of resisting compression.
They can use for fire, building, the manufacture of weapons and tools, furniture, as well as paperwork.
Wood is a heterogeneous, highly porous cellular material. It is made up of cells and the cell walls are made up of roughly 40% cellulose, 15% hemicellulose, and lignin of about (15–30%).
Therefore, we can conclude that a wooden baseball bat is an example of a heterogeneous substance.
Learn more about Heterogeneous substances here:
https://brainly.com/question/24172292?referrer=searchResults
What type of bonds are shown in this diagram?
metallic bonds
covalent bonds
hydrogen bonds
ionic bonds

Answers
Answer:
metallic bonds
Explanation:
atoms in a metallic solid loose their outer electrons and form a regular lattice of positive metallic ions.
The chemical bondings are present between the atoms due to the attractive forces. The bond shown in the diagram represents the metallic bonds. Thus, option A is correct.
What are metallic bonds?A metallic bond is a chemical bonding present due to the electrostatic attractive forces present between the delocalized electrons and the ions of the metals.
The metal produces cations that bond with the electrons delocalized around them. This type of bonding accounts for the malleability and conductivity of the metallic species.
The delocalized electrons are shared by the positively charged metal ions. The cations are largely spread in space. It is seen in the elements of aluminum, magnesium, copper, sodium, zinc, calcium, etc.
Therefore, option A. the metallic bond is seen in the diagram.
Learn more about metallic bonds here:
https://brainly.com/question/20536777
#SPJ2
During an experiment, a student combines 5.0 g of Reactant A with 5.0 g of Reactant B in a flask. The student observes a reaction taking place. It produces a gas, and the flask feels warm to the touch. When the reaction is complete, the student measures the mass of the contents of the flask and determines it to be 9.3 g. The student claims the balance is broken.
Answers
Based on the law of conservation of mass, the apparent loss in mass of the product is due to the gas evolved.
What is the statement of the law of conservation of mass?The law of the conservation of mass states that matter cannot be created or destroyed but can be transformed from one form to another.
The law of conservation of mass is applied in chemical reaction.
Based on the law of conservation of mass, the sum of the masses of the reactants is equal to the sum of the masses of the products in a chemical reaction.
Considering the give reaction:
A student combines 5.0 g of Reactant A with 5.0 g of Reactant B in a flask.
Sum of masses of reactants = 10.0 g
Mass of reactant as shown on the balance after the reaction = 9.3 g
Since the reaction produced a gas, the apparent loss in mass of the product is due to the gas evolved.
Hence, the balance is not broken.
Learn more about law of conservation of mass at: https://brainly.com/question/28218596
#SPJ1
Mass is:
measured in kilograms
measured using a scale
affected by gravity
all of the above
Answers
An experiment shows that 13.88g of
Calcium choride were obtained from the combination of 5g of Ca with chlorine what is the empirical formula of the compound?
Answers
Answer:
CaCl₂
Explanation:
Mass of Calcium chloride = 13.88g
Mass of Ca = 5g
So,
Mass of chlorine = 13.88g - 5g = 8.88g
Now;
Empirical formula of the compound;
The empirical formula is the simplest formula of a compound;
Elements
Ca Cl
Mass 5 8.88
Number of
moles 5/40 8.88/35.5
0.125 0.25
Divide by
the smallest 0.125/0.125 0.25/0.125
1 2
The empirical formula of the compound is CaCl₂
When solving by using the Quadratic Formula, what are the steps to follow? a. factor, find values for a,b,c, subtract c. substitute into the formula, find values for a,b,c, simplify b. simplify, find values for a,b,c, factor d. find values for a,b,c, substitute into the formula, simplify
Answers
Answer:
D!
Explanation:
When solving by using the Quadratic Formula, The steps to follow are to find values for a, b, c, substitute them into the formula, and simplify. The correct option is d.
What are Quadratic Formulas?A quadratic equation is a mathematical equation that shows the equation in an arranged form in which the unknown variables are x and known variables are a, b, and c. Where a is not equal to 0.
The standard quadratic equation
ax² + bx + c = 0
To solve the quadratic equation, first, find the variable value, and substitute the value of a, b, and c. Simplify it and ans solve it and then get the solution. There is another thing in the quadratic equation, which is factorization.
Thus, the correct option is d. find values for a, b, c, substitute them into the formula, and simplify.
To learn more about Quadratic Formulas, refer to the link:
https://brainly.com/question/11540485
#SPJ2
What is the limiting reagent in the reaction of 0.150 g of salicylic acid with 0.350 mL of acetic anhydride (d=1.082 g/mL)? Show your work.
Answers
The limiting reagent for the reaction between 0.150 g of salicylic acid and 0.350 mL of acetic anhydride is salicylic acid, C₇H₆O₃
How do i determine the limiting reagent?First, we shall determine the mass of the acetic anhydride. Details below:
Volume of acetic anhydride = 0.350Density of acetic anhydride = 1.082 g/mLMass of acetic anhydride =?Mass = density × volume
Mass of acetic anhydride, C₄H₆O₃ = 1.082 × 0.350
Mass of acetic anhydride, C₄H₆O₃ = 0.3787 g
Finally, we shall determine the limiting reagent. Details below:
C₇H₆O₃ + C₄H₆O₃ -> C₉H₈O₄ + CH₃COOH
Molar mass of C₇H₆O₃ = 138.121 g/molMass of C₇H₆O₃ from the balanced equation = 1 × 138.121 = 138.121 g Molar mass of C₄H₆O₃ = 102.09 g/molMass of C₄H₆O₃ from the balanced equation = 1 × 102.09 = 102.09 gFrom the balanced equation above,
138.121 g of C₇H₆O₃ reacted with 102.09 g of C₄H₆O₃
Therefore,
0.150 g of C₇H₆O₃ will react with = (0.150 × 102.09) / 138.121 = 0.11089 g of C₄H₆O₃
We can see from the above that only 0.11089 g of acetic anhydride, C₄H₆O₃ out of 0.3787 g is needed to react with 0.150 g of salicylic acid, C₇H₆O₃
Thus, the limiting reagent is salicylic acid, C₇H₆O₃
Learn more about limiting reactant:
https://brainly.com/question/11587316
#SPJ1
which gas is fossil fuel
Answers
Answer:
methane
Explanation: methane is obtained from the decaying of flora and fauna mostlyunder damp
The isotope Ti-48 is produced by the alpha decay of which of the following:
a) ⁵³Mn
b) ⁵⁴Cr
c) ⁵³V
d) ⁵⁴V
e) ⁵²Cr
Answers
Answer:
e) ⁵²Cr
Explanation:
The general form of alpha decay is as follows:
\(\boxed{ ^A_ZX \ \ \rightarrow \ \ ^{A - 4} _{Z - 2} \ Y \ \ + \ \ ^4_2 \alpha}\).
From this, we can see that during alpha decay, the mass number decreases by 4 and the atomic number decreases by 2.
Therefore, we need to find a nucleus that has 4 more nucleons (i.e., a mass number that is 4 more) than that of Ti-48, which is 48 + 4 = 52.
The only option with a nuclear number of 52 is ⁵²Cr, and therefore, Ti-48 is produced by the alpha decay of ⁵²Cr.
Draw a line to connect the following terms to their definition.

Answers
1 - C
2 - A
3 - B
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
The sink-float method is often used to identify the type of glass material found at crime scenes by determining its density.Several different types of glass of known density are placed into solutions of varying densities. Determine whether each glasspiece will sink, float, or do neither when immersed in the given solution.Glass that will sinkGlass that will floatGlass that will not sink or floatalkali zinc borosilicate with a density of2.57 g/mL in a solution with a densityof 2.46 g/mLsoda borosilicate with a density of 2.27 g/mLin a solution with a density of 2.62 g/mLalkali strontium with a density of 2.26 g/mL ina solution with a density of 2.34 g/mLpotash borosilicate with a density of2.16 g/mL in a solution with a densityof 2.16 g/mLpotash soda lead with a density of 3.05 g/mLin a solution with a density of 1.65 g/mLAnswer Bankterms of usecontac
Answers
Answer:
Glass that will sink: alkali zinc borosilicate with a density of2.57 g/mL in a solution with a density of 2.46 g/mL; potash soda lead with a density of 3.05 g/mL in a solution with a density of 1.65 g/mL
Glass that will float: soda borosilicate with a density of 2.27 g/mL in a solution with a density of 2.62 g/mL; alkali strontium with a density of 2.26 g/mL in a solution with a density of 2.34 g/mL
Glass that will not sink or float: potash borosilicate with a density of2.16 g/mL in a solution with a density of 2.16 g/mL
Explanation:
The density of an object is a ratio of the mass (or quantity of matter in the object) to its volume.
Mathematically, density = mass/ volume.
The more dense an object, the higher will be its dense. Density can be thought of as a comparison of how heavy objects having the same are. Objects with a higher density, are heavier than objects with a lower density. For example, between equal volumes of air and water, water is heavier.
The density of solid object will determine whether it will float, sink or neither float nor sink in a given liquid. The conditions for floating of objects in a liquid is given as follows:
Density of solid > Density of liquid : solid will sink
Density of solid = Density of liquid : solid will neither sink nor float
Density of solid < Density of liquid : solid will float
Using the above criteria to analyze the given glass materials in their respective liquids:
Glass that will sink: alkali zinc borosilicate with a density of2.57 g/mL in a solution with a density of 2.46 g/mL; potash soda lead with a density of 3.05 g/mL in a solution with a density of 1.65 g/mL
Glass that will float: soda borosilicate with a density of 2.27 g/mL in a solution with a density of 2.62 g/mL; alkali strontium with a density of 2.26 g/mL in a solution with a density of 2.34 g/mL
Glass that will not sink or float: potash borosilicate with a density of2.16 g/mL in a solution with a density of 2.16 g/mL
A piece of metal is heated to 250 degrees then placed in a bucket of water with an initial temperature of 15 degrees. After 10 minutes, the system reaches a point of thermal equilibrium. The water is now 22 degrees. Which of the following is the most likely temperature of the piece of metal?
Answers
Answer: 22 degrees
Explanation:
Most of the information in this question is put there to throw you off- the only important piece of information here is the final temperature of the water.
In a system of say 2, like in this example, whichever object has more energy in the form of heat will transfer that heat to the object with lesser heat if there is an available path to transfer heat between them. Once the two objects are at the same temperature, however, heat can not be transferred from one to another since neither object has more heat than the other to transfer- this is thermal equilibrium.
This question tells us that the system is at thermal equilibrium- when both objects are at the same temperature. Given the temperature of one object, the water, we then know the other object, the metal is the same temperature.
Part C Before you begin, keep in mind these two points: The timer runs fast, so the minutes go faster than actual minutes. The temperature will rise during the experiment. If the temperature gets very high, lower it to around 300 K. Follow these steps, and then record your observations: Locate the orange reset button on the bottom right side of the screen. Press reset to start the reaction over. Drag the top of the ruler upward until it reaches the 40 mark. Drag the left platform upward until the top of the platform coincides with the 30 mark on the ruler. Toggle the blue play/pause button to the play position at the bottom of the screen to ensure that the reaction doesn’t start before you’re ready. Add 50 A particles, and press the play button on the bottom. Immediately start the timer using the play button on the blue box. At every minute on the timer, pause the simulation and record the number of A and B particles.

Answers
The given instructions outline the steps to follow in a simulation or experiment involving particles A and B. The purpose is to observe and record the number of A and B particles at each minute on the timer. The exact observations and data collection will depend on the specific simulation or experiment being conducted.
Locate the orange reset button: Find the reset button on the bottom right side of the screen and press it to start the reaction over. This ensures that the previous data is cleared and the experiment begins from the initial state.
Drag the ruler: Use the mouse or touch screen to drag the top of the ruler upward until it reaches the 40 mark. This step sets the reference point for measuring the positions of the particles.
Adjust the left platform: Drag the left platform upward until the top of the platform aligns with the 30 mark on the ruler. This step positions the platform for the particles to interact within the desired range.
Toggle the blue play/pause button: Locate the blue play/pause button at the bottom of the screen. Make sure it is in the play position to prevent the reaction from starting before you are ready. This allows you to control the timing of the experiment.
Add 50 A particles: Use the appropriate tool or feature to add 50 A particles to the simulation or experimental setup. This step ensures that the initial condition includes a specific number of A particles.
Start the timer and record observations: Press the play button on the blue box to start the simulation or experiment. Immediately start the timer using the play button on the timer itself. At every minute on the timer, pause the simulation and record the number of A and B particles. This step allows you to track the changes in the particle population over time.
Note: The specific details and actions may vary depending on the simulation or experiment being conducted. It is important to follow the given instructions accurately and record the observations as instructed.
For more such questions on experiment , click on:
https://brainly.com/question/26117248
#SPJ8
A pregnant client experienced preterm labor at 30 weeks gestation. Upon assessing the client the nurse finds that the newborn is at risk of having cerebral palsy. Which medication administration should the nurse perform to prevent cerebral palsy in the newborn?
A. Calcium gluconate.
B. Magnesium sulfate.
C. Glucocorticoid drugs.
D. Antibiotic medications
Answers
The medicine that has to be given by the nurse to prevent cerebral palsy in the new-born is magnesium sulphate.
The term "cerebral palsy" refers to a collection of conditions that impair mobility and posture development and are thought to be caused by non-progressive abnormalities. Insults that caused cerebral palsy are thought to have happened during foetal development or early childhood. A significant risk factor for cerebral palsy is preterm delivery, and that risk rises sharply as gestational age decreases. Right now, 25% of all new occurrences of cerebral palsy are caused by babies delivered before 34 weeks of pregnancy. Cerebral palsy risk is higher when there are several pregnancies. MgSO4 was infused continuously at a rate of 2 g/h for up to 12 hours after a 6 g loading dose. Given before delivery, magnesium sulphate is known to regulate the vasculature and minimize hypoxia effects by neutralising cytokine or excitatory amino acid damage, which may lower the risk.
To know more about pregnancy visit:
https://brainly.com/question/26430164
#SPJ4
A 200 N force is applied to an object, which then accelerates at 2 m/s². What is the mass of the object?
Answers
The mass of the object that is acted on by a force of 200 N is 100 kg
What is mass?Mass can be defined as the quantity of matter a body contains.
To calculate the mass of the object, we use the formula below.
Formula:
m = F/a................ Equation 1Where:
m = Mass of the objectF = Force of the objecta = Acceleration due to gravityFrom the question,
F = 200 Na = 2 m/s²Substitute these values into equation 1
m = 200/2m = 100 kgHence, the mass of the object is 100 kg.
Learn more about mass here: https://brainly.com/question/19385703
#SPJ1
What is the limiting reactant in the following equation? How much Fe2O3 will be produced if 2.1 g of Fe with 2.1 g of O2?
4 Fe + 3O2 —> 2Fe2O3
Answers
Answer:
Fe is limiting, and it will produce .0188 mols of Fe2O3
Explanation:
after you convert both Fe and O2 to mols by using their molar mass, you see there is less Fe than O2 so that is your limiting reactant. To find the amount of Fe2O3 you devide the limiting reactant by it's coefeciant (4) then multiply it by the products coefficant (2). Let me know if you have any questions