Answers
The compounds in order of decreasing solubility in water are written as 1-butanthiol < 1-butanol < 1,2-propanediol. The form is a<b<c.
The maximum amount of a chemical that will dissolve in a given amount of solvent at a given temperature is referred to as its solubility. In other words, solubility refers to a substance's or a solute's capacity to combine with another substance or solvent to produce a solution. The solubility of the substance is determined by the ability of the substance to form hydrogen bonding with water and the size of the alkyl group.
Here, 1-butanthiol has the thiol sulfhydryl group (SH) group in it. This forms a weaker H-bond than the other given compounds. Therefore, this is the least soluble. 1-butanol has less OH group and has a longer alkyl chain. Therefore, this one is less soluble than 1,2-propanediol but not less than 1-butanol. Therefore, the solubility is written as 1-butanthiol < 1-butanol < 1,2-propanediol. This is written as abc.
To know more about solubility:
https://brainly.com/question/28319191
#SPJ4
Related Questions
Using the ionization constants (ka and kb) tables in your online textbook's appendix, calculate the ph of a 1.32 m ammonium chloride (nh4cl) aqueous solution. (enter the numerical value in the space provided below.)
Answers
The pH of the 1.32 M ammonium chloride (NH4Cl) aqueous solution is 9.12.
To determine the pH of the 1.32 M ammonium chloride (NH4Cl) aqueous solution we need the values of ionization constants (ka and kb) and the concentration of ammonium chloride which is 1.32 M.
The values of ionization constants (ka and kb) are
Ka of NH4+ = 5.6 x 10-10
Kb of Cl- = 1.0 x 10-7
Calculate the concentrations of NH4+ and Cl- in the solution.
NH4+ = 1.32 M
Cl- = 1.32 M
Calculate the equilibrium concentrations of H3O+ and OH- using the ionization constants.
Ka = [H3O+][NH4+]/[NH4+]
[H3O+] = Ka x [NH4+] = (5.6 x 10-10)(1.32) = 7.37 x 10-10
Kb = [OH-][Cl-]/[Cl-]
[OH-] = Kb x [Cl-] = (1.0 x 10-7)(1.32) = 1.32 x 10-7
Calculate the pH of the solution.
pH = -log[H3O+] = -log(7.37 x 10-10) = 9.12
The pH of the 1.32 M ammonium chloride (NH4Cl) aqueous solution is 9.12.
For more questions like pH calculation click the link below:
https://brainly.com/question/9278932
#SPJ4
Discuss the large-scale environmental impacts of soil pollution caused by industrial wastes.
Answers
Answer: Industrial processes including mining and manufacturing historically have been leading causes of soil pollution. Industrial areas typically have much higher levels of trace elements and organic contaminants. This is due to intentional and unintentional releases from industrial processes directly into the environment, including to the soil, adjacent water bodies, and the atmosphere.
Explanation:
Perform the calculation and record the answer with the correct number of sig figs
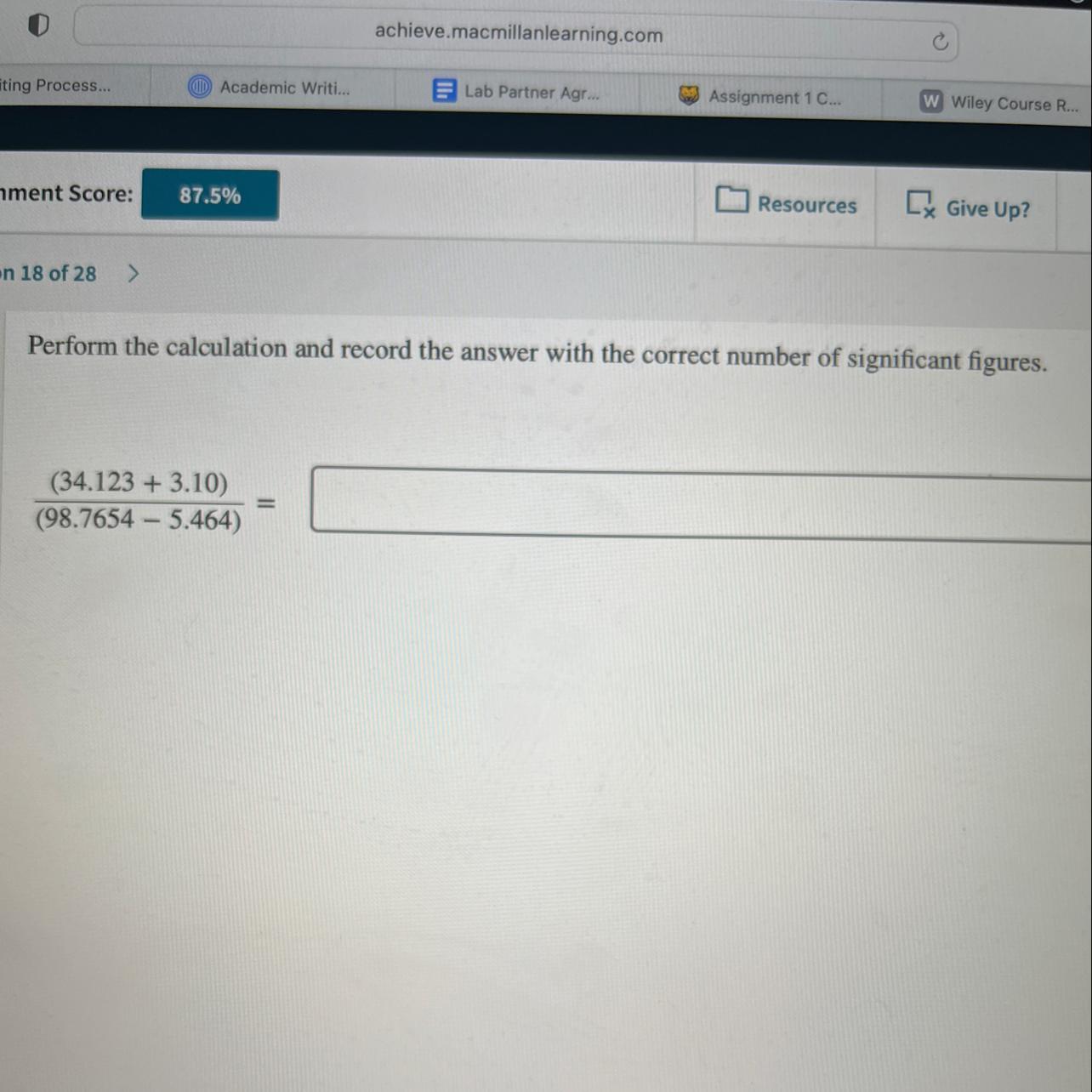
Answers
Answer:
0.399
Explanation:
Sig Fig rules
When initially set up, in which direction does the thermal energy between the flasks flow?
A
Thermal energy flows from the flask on the left to the flask on the right.
B
Thermal energy flows from the flask on the right to the flask on the left.
C
Thermal energy does not flow between the two flasks.
D
Thermal energy flows equally between the two flasks.

Answers
Answer:
How you going to delete my answer even though it was right but dont delete his answer bro didnt even say nun
Explanation:
Answer D
Thermal energy flows from the flask on the left to the flask on the right as energy is transferred from higher to lower temperature.
What is thermal energy?
Thermal energy is defined as a type of energy which is contained within a system which is responsible for temperature rise.Heat is a type of thermal energy.It is concerned with the first law of thermodynamics.
Thermal energy arises from friction and drag.It includes the internal energy or enthalpy of a body of matter and radiation.It is related to internal energy and heat .It arises when a substance whose molecules or atoms are vibrating faster.
These vibrating molecules and atoms collide and as a result of which heat is generated in a substance , more the collision of particles , higher is the thermal energy.
Learn more about thermal energy,here:
https://brainly.com/question/3022807
#SPJ2
D » » DI
Which applies to fusion? Check all that apply.
involves the splitting of nuclei
takes place in the Sun
releaſes radiation as a waste product
occurs in nuclear power plants and is used to generate electricity
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
I’m looking for fusion not fission
Answers
Answer:
The correct answer is -
takes place in the Sun
plays a role in the production of essentially all elements heavier than helium
releases large amounts of energy
Explanation:
Fusion reaction takes place when two or more small atomic nuclei come in close proximation for a longer time so the nuclear force pulling them together and form into heavier molecules than helium and releases a huge amount of energy by this process.
A great example of this fusion reaction is the sun where nuclear fusion takes place inside the core of the sun and result in a huge amount of release as it is an exothermic reaction.
Answer:
2, 5, 6Explanation
EDGE2021
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
calculate the number of moles in 8.45 x 1023 C atoms
Answers
Answer:
1.40
Explanation:
8.45×\(10^{23}\) × \(\frac{1 mol}{6.022x10x^{23} }\) = 1.40318 ≈ 1.40 mol
find one word for (movement of animals from one place to another)
Answers
Explanation:
احبك كثيرا اتمنى ذلك أن أقول
what's common to H20,HF and NH3
Answers
Answer:
They all contain sone hydrogen atoms.
Explanation:
H is hydrogen doi
Question #37.P.2A.2
Which statement about the periodic table is true?
a. Elements in the same column share similar properties.
b. Elements in the same row share similar properties.
c. Elements on the left have a larger nucleus than elements on the right.
d. Elements at the top of each column have the highest atomic mass in that column.
Answers
This is because they have the same number of valence electrons, so they react and bond similarly.
When two oppositely charged particles are closer together, they have a ___________ attraction toward one another than if they are farther apart.
Answers
When two oppositely charged particles are closer together, they have a stronger attraction toward one another than if they are farther apart.
The electrostatic force, which is the force of attraction or repulsion between two charged particles, is the cause of this attraction. The magnitude of the electrostatic force between two charged particles is inversely proportional to the square of the distance between them and directly proportional to the product of their charges.
The inverse square law states that as the distance between two charged particles moves closer together, the electrostatic force between them grows as a result of a smaller denominator. Therefore, the stronger the attraction between two oppositely charged particles will be the closer they are.
Learn more about electrostatic force at:
brainly.com/question/12992080
#SPJ1
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
Which state of matter consists of particles that cannot be compressed and form a definite shape?
Answers
Solid state
Explanations:Matter is any has weight and occupies space. The states of matter that we have are the solid, the liquid, gaseous state and plasma.
Solid states are closely packed together and the particles only vibrate about their mean free space compared to liquid and gases that are loosely packed.
The solid state particles are therefore known to form a definite shape and cannot be compressed due to the strong intermolecular attractions that exists between its particles.
Which of the following relations is correct for exothermic and endothermic reactions?
Answers
Answer:
pa helpppp
Explanation:
pleseee need answerrrr
The correct relations for exothermic and endothermic reactions from the given relations is H Products > H Reactants in exothermic reactions. Hence, Option (D) is Correct.
What is Exothermic Reaction ?
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change ΔH⚬ is negative." Exothermic reactions usually release heat.
Therefore, The correct relations for exothermic and endothermic reactions from the given relations is H Products > H Reactants in exothermic reactions. Hence, Option (D) is Correct.
Learn more about reaction here ;
https://brainly.com/question/17434463
#SPJ2
Part 2 The student wanted to know if the value obtained from their experiment (part 1) is similar to that calculated using average bond enthalpy data.
a) Using the balanced equation and the data in the table below, calculate the theoretical enthalpy of combustion.
Note: you will need to include the enthalpy of vaporisation for the liquid components which are also given.
C₂H5OH()+302(g) → 2CO2(g) + 3H₂O(1)
Average Bond Enthalpies (kJ mol-¹)
C-H 412
C-C 348
C-O 358
O=O 496
C=O 743
O-H 463
Enthalpy of Vaporisation (kJ mol-¹)
Ethanol 42.5
Water 41
Answers
-1113.5kJ is the theoretical enthalpy of combustion.
What makes energy different from enthalpy?
The entire amount of heat energy that is either absorbed or released in a thermodynamic system is measured by enthalpy. Internal energy denotes all of the potential or moving energy present in a thermodynamic system.
Enthalpy of combustion is the term used to describe the change in a system's enthalpy that occurs when one mole of a substance fully burns in oxygen or air at a specific temperature.
C₂H5OH()+302(g) → 2CO2(g) + 3H₂O(1)
Reactants:
5 C-H : 5*412
1 C-C : 348
1 C-O: 358
3 O=O: 3* 496
1 O-H: 463
Products:
2*2 C=O : 4*743
2*3 O-H: 6*463
Enthalpy of Vaporization (kJ mol-¹) for :
Ethanol 42.5
Water 41
Enthalpy of combustion : (5*412 + 348 + 358 + 3* 496 + 463 + 42.5) - ( 3*41 + 4*743 + 6*463)
: -1113.5kJ
To learn more about enthalpy use :
https://brainly.com/question/5374936
#SPJ1
How many protons are in an element with an atomic number of 8 and mass number of 18
Answers
Answer:8
Explanation:
Atomic number = 8
Mass number = 18
The number of protons and the number of electrons is equal to the atomic number.
The number of neutrons in the nucleus is given by the difference in mass number and atomic number.
So, number of protons are 8
number of electrons = 8
number of neutrons = 18 - 8 = 10
The primary role of coenzyme A in aerobic respiration is to
A. speed up the citric acid cycle.
B. transport electrons to FAD
C. kick off the transport electron cascade.
D. activate acetyl CoA and other molecules in the citric acid cycle.
Answers
Answer:
I could be wrong but i believe the answer is D. It was not B when i took the test
Explanation:
How many molecules are in 82.93 moles of N205?
Answers
Answer:
2.41 molecules.
Explanation:
hope this helps!
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Task:
For each "station", click on the link. You should describe the initial appearances and observations of the
reaction during and after. Using your observations, determine if the change is a physical or chemical change.
Station #1: Lead Nitrate and Potassium lodide solutions. Shower of yellow
QUESTION/OBSERVATION
INITIAL APPEARANCE (what does the
substance look like in the beginning)
Answers
The expected observations for the chemical reaction involving lead nitrate and potassium iodide are as follows as per theory.
INITIAL APPEARANCE:Before the reaction, you'd have two separate solutions:
Lead Nitrate solution: This is typically a clear, colorless solution.
Potassium Iodide solution: This is also usually a clear, colorless solution.
REACTION OBSERVATIONS:
As soon as you combine these two solutions, a chemical response takes place, resulting in the almost instantaneous development of a yellow precipitate. Lead iodide is a substance that cannot be dissolved in water.
FINAL APPEARANCE:
The final mixture would have a yellow precipitate (lead iodide) suspended in the solution.
The reaction leads to the formation of lead iodide, a substance with distinctive properties, suggesting a chemical change. The presence of this novel compound is indicated by the yellow hue of the precipitate.
Read more about chemical change here:
https://brainly.com/question/1222323
#SPJ1
A vessel contains 2.00 mol of He, 4.50 mol of Kr, and 0.50 mol of N2 gases. If the partial pressure of He is 0.120 atm, what is the total pressure inside the vessel?
Answers
Considering the Dalton's partial pressure, the total pressure inside the vessel is 0.42 atm.
Dalton's partial pressureDalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T}\)= P₁ + P₂ + ... + Pₙ
where n is the amount of gases present in the mixture.
This relationship is due to the assumption that there are no attractive forces between the gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture. So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
\(P_{A}\)=\(x_{A}\)\(P_{T}\)
Total pressure inside the vesselIn this case, you know:
Amount of moles of He= 2 molesAmount of moles of Kr= 4.50 molesAmount of moles of N₂= 0.50 molesTotal amount of moles= Amount of moles of He + Amount of moles of Kr + Amount of moles of N₂= 2 moles + 4.50 moles + 0.50 moles= 7 molesPartial pressure of He= 0.120 atmThe partial pressure of gas He can be expressed as:
\(P_{He}\)=\(x_{He}\)\(P_{T}\)
Then, calculate the mole fraction of He as:
\(x_{He}\)= Amount of moles of He÷ Total amount of moles
\(x_{He}\)= 2 moles÷ 7 moles
\(x_{He}\)= 2/7
the total pressure can be calculated as:
0.120 atm= 2/7×\(P_{T}\)
0.120 atm÷ 2/7=\(P_{T}\)
0.42 atm= \(P_{T}\)
Finally, the total pressure is 0.42 atm.
Learn more about Dalton's partial pressure:
brainly.com/question/14239096
brainly.com/question/25181467
brainly.com/question/14119417
#SPJ1
-physical and chemical methods
of monitoring the rate of
chemical reaction
Answers
1. Measuring changes in concentration: By measuring changes in the concentration of reactants or products, you can calculate the rate of the reaction. This can be done using spectrophotometry or titration.
2. Temperature measurement: The rate of a reaction is often temperature-dependent. By monitoring the temperature of the reaction mixture over time, you can determine the rate at which the reaction is proceeding.
3. Gas volume measurement: In reactions that produce or consume gases, measuring the volume of gas generated or consumed over time can give you an idea of the reaction rate. This can be done using a gas syringe or a gas burette.
4. Conductivity measurement: If the reaction involves the transfer of ions, measuring the conductivity of the reaction mixture can provide information about the reaction rate.
5. Pressure measurement: In some reactions, the pressure of the reaction mixture changes as the reaction proceeds. Measuring the pressure change over time can give you an idea of the reaction rate.
These are just a few examples of physical and chemical methods for monitoring the rate of a chemical reaction. The choice of method will depend on the nature of the reaction and the information you need to obtain.
Physical methods include monitoring temperature, pressure, and color change. Chemical methods include titration and gas analysis.
What are methods of monitoring chemical reaction?Monitoring the rate of chemical reactions is important to understand the kinetics of the reaction and optimize the reaction conditions. Physical and chemical methods are used for this purpose.
Physical methods include measuring the change in temperature, volume, and pressure of the reactants and products with time. The rate of reaction can be calculated from the rate of change of these parameters.
Chemical methods include monitoring the concentration of reactants and products with time. This can be done by techniques such as spectroscopy, chromatography, and electrochemistry. These methods are often more accurate and precise than physical methods.
Learn more about chemical reaction, here:
https://brainly.com/question/29039149
#SPJ2
1
Which of the following is a true statement
about atomic nuclel?
A They are made up of protons, neutrons, and electrons
B They have
net positive charge
C Every atomic nucleus has the same number of particles
D
The atomic nucleus has no relation to an atom's mass
Answers
Answer:
I believe the answer is B
Explanation:
A nucleus of an atom has protons and neutrons. We know that a proton has a charge of +1 , while a neutron has no charge, or 0 . Therefore, the nucleus of an atom will always have a positive charge.
A 0.205 g sample of CaCO3 (Mr = 100.1 g/mol) is added to a flask along with 7.50 mL of 2.00 M HCl. CaCO3(aq) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g) Enough water is then added to make a 125.0 mL solution. A 10.00 mL aliquot of this solution is taken and titrated with 0.058 M NaOH. NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) How many mL of NaOH are used?
Answers
The required volume of sodium hydroxide is 15 mL.
What is concentration?The term concentration refers to the amount of substance present. It is the quotient of the number of moles and volume of solution.
We have the reaction; 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Number of moles of CaCO3 = 0.205 g / 100.1 g/mol = 0.0020 moles
Number of moles of HCl = 2.00 M * 7.50/1000 = 0.015 moles
Since 2 moles of HCl reacts with 1 mole of CaCO3
x moles of HCl reacts with 0.0020 moles
x = 0.004 moles of HCl
This means that HCl is in excess by the amount 0.011 moles.
Now consider the reaction; NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq)
Concentration of HCl = 0.011 moles/0.125L = 0.088 M
From;
CAVA/CBVB = na/nb
CAVAnb = CBVBna
VB = CAVAnb/CBna
VB = 0.088 M * 10mL * 1/0.058 M * 1
VB = 15 mL
Learn more about concentration: https://brainly.com/question/3045247
Drag the tiles to the correct boxes to complete the pairs. Not all tiles will be used.
Match each SI unit to the quantity it measures.
Answers
The SI unit to the quantity it measures are:
mass - kilogram, gramtemperature - kelvintime - second, nanosecondelectric current - ampereWhat is SI unit used for?Mass: The mass of an object is a measure of its amount of matter. The SI unit of mass is the kilogram (kg) or gram (g).
Temperature: Temperature is a measure of the average kinetic energy of the particles in a substance. The SI unit of temperature is the kelvin (K).
Time: Time is a measure of the interval between two events. The SI unit of time is the second (s).
Electric current: Electric current is a measure of the flow of electric charge. The SI unit of electric current is the ampere (A).
Find out more on SI unit here: https://brainly.com/question/16393390
#SPJ1
Complete question:
Drag the tiles to the correct boxes to complete the pairs. Not all tiles will be used.
Match each SI unit to the quantity it measures.

What state
of matter
exists in
area B?
A. gas
B. liquid
C. solid
Pressure
(atm)
61
6543210
0
50 100 150 200
Temperature (°C)
Answers
Considering the phase diagram, the state of matter that exists in area B is gas.
The correct option is A.
What is a phase diagram?A phase diagram is a graphical representation that shows the conditions of temperature and pressure at which different phases or states of a substance exist.
The axes of a phase diagram typically represent temperature (usually on the horizontal axis) and pressure (usually on the vertical axis). The diagram is divided into regions that correspond to different phases, and the lines separating these regions represent phase boundaries.
The point where three phase boundaries meet is known as the triple point, which represents the temperature and pressure at which all three phases can coexist in equilibrium.
Learn more about phase diagrams at: https://brainly.com/question/28097253
#SPJ1

Substance in which state of matter have most space between particles
Answers
Answer:
to answer your question "which state of matter have most space between particles"
gas is your best answer
Explanation:
when in gas form..particles gain energy and move further apart
for solids particles are fixed (stays in one spot) and vibrates and for liquids particles gain energy but doesn't have enough space to move around freely..so gas is you best answer
Arrange the highlighted bonds in the table below and decreasing order of polarity. That is, pick one for the most polar bond, pick two for the next most polar bond, and so on

Answers
For this exercise we need the polarity of the bonds, for that, we need the electronegativity of the elements from the bonds.
These are nonmetals, so we have covalent bonds.
The 3 of the highlighted bonds are covalent.
-----------------------------------------------------------------------
-C-C-
We calculate the polarity as a subtraction:
Polarity (-C-C-) = 0 = 2.5 - 2.5
2.5 is the electronegativity for C
-----------------------------------------------------------------------
-C-O-
Polarity (-C-O-) = 3.5 (O) - 2.5 (C) = 1.0
-----------------------------------------------------------------------
-C-F-
Polarity (-C-F-) = 4.0 (F) - 2.5 (C) = 1.5
------------------------------------------------------------------------
The electronegativities can be obtained from the Pauling scale.
Now the answer:
Decreasing order of polarity:
-C-F (the most) = 1.5 > -C-O-(the next) = 1.0 > -C-C- (the less) = 0
Which ketone in each pair is more reactive?
a. 2-heptanone or 4-heptanone,
b. bromomethyl phenyl ketone or chloromethyl phenyl ketone
Answers
Answer:
a. 2-heptanone is more reactive than 4-heptanone
b. chloromethyl phenyl ketone is more reactive than bromomethyl phenyl ketone
Explanation:
The reactivity of the carbonyl compound (ketone ) is affected by the steric effect. The steric effect is a hindrance that occurs in the structure or reactivity of a molecule, which is affected by the physical size and the proximity of the adjacent parts of the molecule.
Between 2-heptanone or 4-heptanone, 2-heptanone is more reactive than 4-heptanone. This is because 2-heptanone is less affected by the steric hindrance, unlike the 4-heptanone.
Similarly, the reactivity of the carbonyl compound (ketone) is also affected by the polarity on the carbon compound, which is associated with how electronegative the substituent attached is to the carbonyl compound. From the periodic table, the electronegativity of the Halogen family decreases down the group. Therefore chlorine is more electronegative than bromine.
As such, chloromethyl phenyl ketone is more reactive than bromomethyl phenyl ketone.
In some sheep, the presence of horns is produced by an autosomal allele that is dominant in males and recessive in females.A horned female is crossed with a hornless male. One of the resulting F1 females is crossed with a hornless male. What proportion of the male and female progeny from this cross will have horns?(5 marks)
Answers
Answer:
1/2 f1 will cross
Explanation:
answer it