Archeologists can determine the age of an artifact made of wood or bone by measuring the amount of the radioactive isotope ¹⁴C present in the object. The amount of this isotope decreases in a first-order process. If 15.5% of the original amount of ¹⁴C is present in a wooden tool at the time of analysis, what is the age of the tool? The half-life of ¹⁴C is 5730 yr.
Answers
Archeologists can determine the age of an artifact made of wood or bone by measuring the amount of the radioactive isotope. The age of the tool is 15, 396.51 years.
What is the half-life?Half-life is the time of the decay of half of the substance.
After its second half-life will have 50% of the 0.5A So, the mass will be 0.25A. So, the percentage of A, is given by:
A = (0.5)n
Where n is quantitative of half-life. So, for 15.5% of C, or 0.155:
0.155 = (0.5)n
Applying log on both sides of the equation:
log 0.155 = log (0.5)n
n log(0.5) = log(0.155)
-0.301n = - 0.809
n = 2.687 half-life
If one half-life is 5,730 yr, than 2.419 will be:
2.687 x 5730 = 15, 396.51 years
Thus, the age of the tool is 15, 396.51 years.
To learn more about the half-life, refer to the link:
https://brainly.com/question/24710827
#SPJ4
Related Questions
If the rate law for the reaction 2A + 3B → products is second order in A and zero order in B, then the rate law is rate =. A) k[A][B] B) k[A]2[B]3 C) k[A][B]2 D) k[A]2 E) k[A]2[B]2
Answers
The rate law for a chemical reaction describes the relationship between the concentrations of reactants and the rate of the reaction. In the given reaction, 2A + 3B → products, the rate law is second bin A and zero order in B.
The rate of the reaction depends on the concentrations of the reactants raised to the powers of their respective orders. Since the reaction is second order in A, the rate is proportional to [A]^2. Similarly, since the reaction is zero order in B, the rate is not influenced by the concentration of B.
Therefore, the correct rate law for this reaction is option D) k[A]^2, where k is the rate constant. This means that the rate of the reaction is directly proportional to the square of the concentration of A.
The other options (A, B, C, and E) do not accurately reflect the given rate law. Option A suggests that the rate is first order in B, which is not consistent with the given zero order. Option B suggests different orders for A and B, which is not the case. Option C suggests a second order dependence on B, which is not consistent with the given zero order. Option E suggests a combined order of 4, which is not consistent with the given second order for A and zero order for B. Therefore, option D) k[A]^2 is the correct rate law.
Know more about Rate law here:
https://brainly.com/question/30379408
#SPJ11
What mass of water freezes if 5.43 kJ oh heat are released in the process
Answers
Answer:
16.3 g
Explanation:
got it right on ck
What is the molarity when 25.0 g of the compound NaClO3 is placed in 85.0 mL of solution?
Answers
Answer: Molarity when 25.0 g of the compound \(NaClO_{3}\)is placed in 85.0 mL of solution is 294.12 M.
Explanation:
Given: Mass = 25.0 g
Volume = 85.0 mL (1 mL = 0.001 L) = 0.085 L
Molarity is the number of moles of a substance divided by volume in liter.
Hence, molarity of given solution is calculated as follows.
\(Molarity = \frac{mass}{Volume (in L)}\)
Substitute the values into above formula as follows.
\(Molarity = \frac{mass}{volume (in L)}\\= \frac{25.0 g}{0.085 L}\\= 294.12 M\)
Thus, we can conclude that molarity when 25.0 g of the compound \(NaClO_{3}\)is placed in 85.0 mL of solution is 294.12 M.
A student wants to develop a model that categorizes various plants and animals as either heterotrophs or autotrophs. Which statement provides the BEST criteria for distinguishing which category the various
organisms should be placed within the model?
A
Heterotrophs are multicellular organisms that reproduce sexually, autotrophs are unicellular organisms that reproduce asexually.
B
Heterotrophs can produce their own food from inorganic sources such as carbon dioxide, autotrophs need to consume other organisms in the food chain for sustenance
C
Heterotrophs need to consume other organisms in the food chain for sustenance; autotrophs can produce their own food from inorganic sources such as carbon dioxide
D
Autotrophs are multicellular organisms that reproduce sexually; heterotrophs are unicellular organisms that reproduce asexually.
Answers
What measures are being taken to minimize the threat of green iguana?
Answers
Answer:
use wire nettings
Explanation:
and cages
Acidic
A) is an excess of OH-
B) is an excess of H+ ions
C) when alkali dissociate, anion
D) is the division of chemistry that deals with the transfer of electric charge in chemical reactions
E) loss of electrons yielding a positively charged ion
Answers
An acidic solution can be defined as one that has an excess of H+ ions .So the correct option is option B.
Acidity is a property of a substance that describes its ability to donate hydrogen ions (H+). A substance with a high concentration of H+ ions is considered acidic. In aqueous solutions, the concentration of H+ ions is balanced by the concentration of hydroxide ions (OH-). When the concentration of H+ ions is greater than the concentration of OH- ions, the solution is acidic.
Option A is incorrect because an excess of OH- ions in a solution makes it basic or alkaline, not acidic.
Option C is incorrect because the anion is not directly related to acidity. An anion is a negatively charged ion that is formed when an atom gains one or more electrons.
Option D is incorrect because electrochemistry deals with the transfer of electric charge in chemical reactions. Acidity is a broader concept that involves the concentration of H+ ions in a solution.
Option E is incorrect because the loss of electrons yielding a positively charged ion is called oxidation, which is not directly related to acidity.
Learn more about anion here:
https://brainly.com/question/20781422
#SPJ11
If a rock is heated by metamorphism and the daughter atoms generated by the decay of the radioactive parent atoms migrate out of a mineral that is subsequently radiometrically dated, the date will be _____________ the actual age.
Answers
If a rock is heated by metamorphism and the daughter atoms generated by the decay of the radioactive parent atoms migrate out of a mineral that is subsequently radiometrically dated, the date will be younger than the actual age.
When daughter atoms generated by the decay of radioactive parent atoms migrate out of a mineral, the amount of parent and daughter isotopes present will no longer accurately reflect the time that has passed since the mineral formed. As a result, the radiometric date obtained will be younger than the actual age of the rock. This is why it is important to carefully consider the sample being dated and any potential disturbances it may have undergone during its history.
This leads to an underestimation of the rock's age, making the radiometric date appear younger than the actual age.
learn more about radiometric date
https://brainly.com/question/8831242
#SPJ11
how many total valence electrons are present in a molecule of PCl3 ?
Answers
The total number of valence electrons that are present in a molecule of PCl3 is 26.
PCl3 stands for Phosphorus Trichloride. The molecular structure of PCl3 is trigonal pyramidal. It has three chlorine atoms and one phosphorus atom, which are bonded by three covalent bonds.
In order to determine the total number of valence electrons in PCl3, first we have to Count the valence electrons present in each atom. Phosphorus has 5 valence electrons. Chlorine has 7 valence electrons then Add the valence electrons from each atom.
P = 5 e- (phosphorus has 5 valence electrons)
Cl = 7 e- (chlorine has 7 valence electrons)
Total valence electrons in PCl3 = 5 + 7 × 3 = 5 + 21 = 26.
Therefore, there are 26 valence electrons in a molecule of PCl3.
For more such questions on valence electrons, click on:
https://brainly.com/question/371590
#SPJ11
elements are identified by their atomic number because.
Answers
Answer:
The number of protons in the nucleus determine their atomic number.
Explanation:
Since an element is made up of one type atoms, the atomic number is found in the amount of protons that are found in the nucleus.
which picture best represents a mixture of elements
Answers
Answer: the one thats not there because you didnt put any pictures
In general, what happens when a subscript is found
outside of parentheses?
Answers
Answer:
you multiply it by the other subscripts in the parenthesis.
Explanation:
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place over the next few minutes? Molecules in both the metal and the surrounding air will start moving at lower speeds. Molecules in both the metal and the surrounding air will start moving at higher speeds. The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up. The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
Answer:
last option is correct.
Air molecules surrounding the metal will speed up and metal molecules will slow down
Explanation:
the metal cools when placed in room temperature air which causes surrounding air to heat up.
the speed of molecules slow down for any material which cools and molecules speed increases when any material heats up
(Revision) When magnesium, Mg, form magnesium ions, Mg2+, has it beer
oxidised or reduced? *

Answers
Answer:
it has oxidized because it has has given out 2 ions
Answer:
Oxidised
Explanation:
Mg -------> Mg2+ +2electrons
In this case Mg loses two electrons and loss of electron is oxidation so Mg oxidised.
Gases ___ out and fill their container completely
Answers
Answer: Gases have the lowest density of the three, are highly compressible, and completely fill any container in which they are placed. Gases behave this way because their intermolecular forces are relatively weak, so their molecules are constantly moving independently of the other molecules present. Solids, in contrast, are relatively dense, rigid, and incompressible because their intermolecular forces are so strong that the molecules are essentially locked in place.
Explanation:
Calculate the pH and the equilibrium concentration of S²- in a 6.89x10-2 M hydrosulfuric acid solution, H₂S (aq). For H₂S, Ka1 = 1.0x10-7 and Ka_2 = 1.0×10-1⁹ pH = [S²] = M
Answers
Therefore, the pH and the equilibrium concentration of S²⁻ in a 6.89x10⁻² M hydrosulfuric acid solution are pH = 7.78 and [S²⁻] = 2.31x10⁻¹¹ M.
Hydrosulfuric acid (H₂S) is a weak acid that dissociates in water to produce hydrogen ions (H⁺) and bisulfide ions (HS⁻). H₂S(aq) + H₂O(l) ⇌ H₃O⁺(aq) + HS⁻(aq)
The bisulfide ions (HS⁻) in turn reacts with water to produce hydronium ions (H₃O⁺) and sulfide ions (S²⁻).
HS⁻(aq) + H₂O(l) ⇌ H₃O⁺(aq) + S²⁻(aq) Ka1
= 1.0x10⁻⁷,
Ka2 = 1.0x10⁻¹⁹
To calculate the pH and the equilibrium concentration of S²⁻ in a 6.89x10⁻² M H₂S(aq) solution, we must first determine if H₂S(aq) is a strong or weak acid.
It has Ka1 = 1.0x10⁻⁷, which is a very small value; thus, we can conclude that H₂S(aq) is a weak acid.
To calculate the equilibrium concentration of S²⁻ in a 6.89x10⁻² M H₂S(aq) solution, we need to use the Ka2 value (Ka2 = 1.0x10⁻¹⁹) and a chemical equilibrium table.
[H₂S] = 6.89x10⁻² M[H₃O⁺] [HS⁻] [S²⁻]
Initial 0 0 0Change -x +x +x
Equilibrium (6.89x10⁻² - x) x xKa2 = [H₃O⁺][S²⁻]/[HS⁻]1.0x10⁻¹⁹
= x² / (6.89x10⁻² - x)
Simplifying: 1.0x10⁻¹⁹ = x² / (6.89x10⁻²)
Thus: x = √[(1.0x10⁻¹⁹)(6.89x10⁻²)]
x = 2.31x10⁻¹¹ M
Thus, [S²⁻] = 2.31x10⁻¹¹ M
To calculate the pH of the solution, we can use the Ka1 value and the following chemical equilibrium table.
[H₂S] = 6.89x10⁻² M[H₃O⁺] [HS⁻] [S²⁻]
Initial 0 0 0
Change -x +x +x
Equilibrium (6.89x10⁻² - x) x x
Ka1 = [H₃O⁺][HS⁻]/[H₂S]1.0x10⁻⁷
= x(6.89x10⁻² - x) / (6.89x10⁻²)
Simplifying: 1.0x10⁻⁷ = x(6.89x10⁻² - x) / (6.89x10⁻²)
Thus: x = 1.66x10⁻⁸ M[H₃O⁺]
= 1.66x10⁻⁸ M
Then, pH = -log[H₃O⁺]
= -log(1.66x10⁻⁸)
= 7.78 (rounded to two decimal places)
To know more about concentration visit:
https://brainly.com/question/30862855
#SPJ11
the atomic weight of hydrogen is 1.008 amu. what is the percent composition of hydrogen by isotope, assuming that hydrogen’s only isotopes are 1h and 2d?
Answers
The percent composition of hydrogen by isotope can be calculated based on the relative abundance of each isotope and their respective atomic masses. In this case, hydrogen has two isotopes: 1H and 2D Percent composition = (0.0002 * 2.014 amu) / [(0.9998 * 1.008 amu) + (0.0002 * 2.014 amu)]
To find the percent composition, we need to consider the relative abundance of each isotope. 1H is the most common isotope of hydrogen, with an abundance of approximately 99.98%. Its atomic mass is 1.002D, also known as deuterium, is the less common isotope, with an abundance of approximately 0.02%. Its atomic mass is 2.014 amu.To calculate the percent composition of each isotope, we can use the following formula:Percent composition = (Abundance * Atomic mass) / Average atomic massLet's calculate the percent composition for each isotope:
1HPercent composition = (0.9998 * 1.008 amu) / Average atomic mas2Percent composition = (0.0002 * 2.014 amu) / Average atomic massTo find the average atomic mass, we can use the weighted average formula:Average atomic mass = (Abundance of 1H * Atomic mass of 1H) + (Abundance of 2D * Atomic mass of 2D)Substituting the values, we get:
To know more about hydrogen visit :-
https://brainly.com/question/31018544
#SPJ11
Three safety-related rules concerning the location of machine controls on equipment involving fluid power components.
Answers
1. Ensure Clear and Visible Placement: Machine controls should be located in a position that is easily accessible, visible, and within reach of the equipment operator. Clear and intuitive labeling or color-coding can also be used to enhance visibility and assist in identifying the controls quickly.
2. Provide Adequate Guarding: The machine controls should be positioned in a manner that minimizes the risk of accidental activation or unintended operation. This can be achieved by incorporating appropriate guarding or barriers around the controls to prevent inadvertent contact or interference.
3. Consider Ergonomics and Operator Comfort: When determining the location of machine controls, it is essential to consider ergonomic principles and operator comfort. Controls should be positioned in a way that allows operators to maintain a comfortable and natural posture while operating the equipment. This can help reduce the risk of operator fatigue, musculoskeletal disorders, and errors due to discomfort or awkward reach.
These rules aim to promote operator safety, minimize the potential for accidents, and ensure efficient and effective control of equipment involving fluid power components.
To know more about musculoskeletal disorders.
https://brainly.com/question/30279097
#SPJ11
What does it mean to neutralize a chemical solution?
Answers
Answer:
A neutralized solution in chemistry refers to the reaction between an acid and base that results in a neutral balance, or a measure of 7 on the pH scale.
Explanation:
2. His_________would take place tomorrow. (bury)
3.His________of words is good. (choose)
4. I received the gift with_______(please)
Answers
Answer:
1. burial
2. Choice
3. pleasure
Explanation:
Mark brainliest! Please!
0.22g of carbon dioxide are dissolved in 400 cm3 of pure water.
Calculate the concentration in mol/dm3 of the
solution produced.
Answers
Answer:
0.0125mol/dm³
Explanation:
Given parameters:
Mass of carbon dioxide = 0.22g
Volume of water = 400cm³
Unknown:
Concentration in mol/dm³ = ?
Solution:
Concentration is the amount of solute dissolved in a solvent.
The formula is expressed as;
Concentration = \(\frac{number of moles}{volume}\)
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of CO₂ = 12 + 3(16) = 44g/mol
Number of moles = \(\frac{0.22}{44}\) = 0.0005mol
Now,
1000cm³ = 1dm³
400cm³ = \(\frac{400}{1000}\) = 0.4dm³
Insert the parameters and solve;
Concentration = \(\frac{0.005}{0.4}\) = 0.0125mol/dm³
Describe the emission spectrum of hydrogen. Outline how this spectrum is related to the energy levels in the hydrogen atom. (3 marks)
Answers
The emission spectrum of hydrogen is a series of colored lines that are produced when an electron in a hydrogen atom falls from a higher energy level to a lower energy level.
The spectral lines in the hydrogen emission spectrum correspond to different energy transitions within the atom. The energy of a photon is directly proportional to its frequency, so the emission lines correspond to specific frequencies of light. The emission spectrum of hydrogen consists of a series of discrete lines, called the Balmer series, which correspond to specific wavelengths of light emitted when electrons in a hydrogen atom transition from higher energy levels to lower ones.
This emission spectrum is related to the energy levels in the hydrogen atom as follows:
1. When an electron in a hydrogen atom absorbs energy, it jumps to a higher energy level, also known as an excited state.
2. The electron then releases the absorbed energy in the form of a photon when it transitions back to a lower energy level. The energy of the emitted photon corresponds to the difference between the two energy levels involved in the transition.
3. The distinct lines in the emission spectrum represent the specific energy differences between these energy levels, and each line corresponds to a unique transition between two energy levels. In summary, the emission spectrum of hydrogen is a direct result of electrons transitioning between different energy levels in the atom, and the specific wavelengths of light emitted correspond to the energy differences between these levels.
learn more about the emission spectrum of hydrogen here
https://brainly.com/question/29255944
#SPJ11
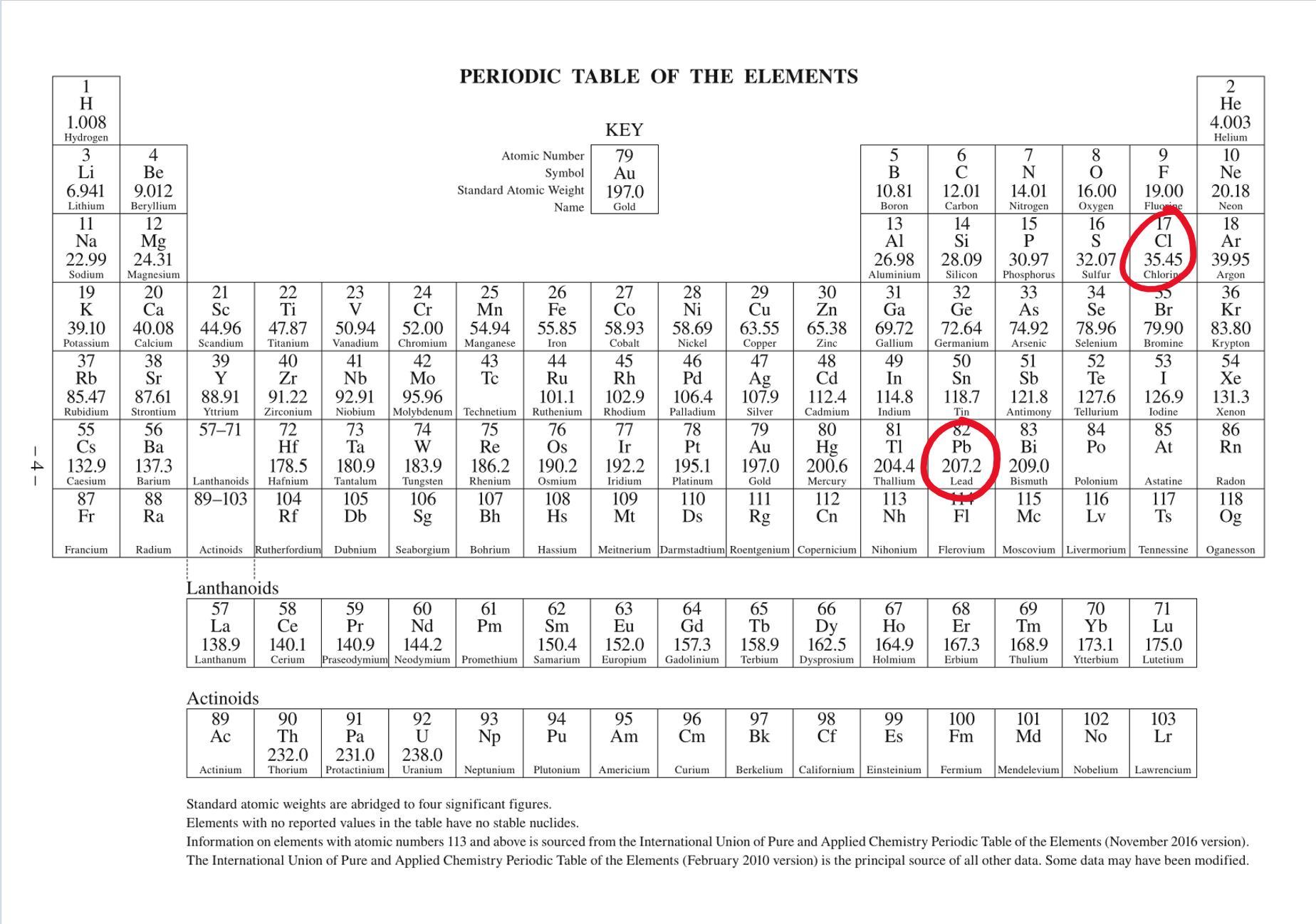
What’s the molar mass of lead(II) chloride
Answers
Answer:
molar mass = 278.1 g/mol
Explanation:
Molar mass is the mass of one mole of element, compound, molecule, etc.
It has the units of grams per mole (g/mol), and in mole calculations, is commonly denote the symbol M.
Lead(II) chloride has the chemical formula:
\(\boxed{\rm PbCl_2}\)
The molar mass of lead chloride, is the total sum of all the individual molar masses of each element. See attached image, standard IUPAC Periodic Table found in data sheets/booklets for most chemistry exams around the world.
Hence molar mass = (207.2)+(35.45)×2 = 278.1 g/mol
To learn more about molar mass:
https://brainly.com/question/30640134
HELP! HELP!
an element with 5 protons, and 8 electrons has an atomic number of?
Answers
Answer:
Explanation: 15
what's the relationship between weight mass and volume
Answers
Which force pulls earth and the sun toward each other?

Answers
Answer:
the correct answer is gravity
Answer:
the gravitational pull so gravity (b)
Titan is a moon of the planet saturn a spacescraft is in a circular orbit around titan at th height of 160 km a small part of the spacecraft falls off and eventually lands on the surface of titan the small part has a mass of 1. 4kg during its fall the small parts loses 0. 303MJof gravitational potential energy calculate the gravitational fieald strenght of titan give anwser to 3 signifacent figures
Answers
To calculate the gravitational field strength of Titan, we can use the formula for gravitational potential energy:
ΔPE = mgh
Where:
ΔPE = Change in gravitational potential energy
m = Mass of the small part (1.4 kg)
g = Gravitational field strength
h = Height (altitude) of the small part above the surface of Titan (160 km or 160,000 meters)
Given that the small part loses 0.303 MJ (mega joules) of gravitational potential energy, we need to convert it to joules:
0.303 MJ = 0.303 × 10^6 J
Now we can rearrange the formula to solve for the gravitational field strength (g):
g = ΔPE / (mh)
Substituting the known values:
g = (0.303 × 10^6 J) / (1.4 kg × 160,000 m)
Calculating this expression gives:
g ≈ 1.090 N/kg
Therefore, the gravitational field strength of Titan is approximately 1.090 N/kg (to 3 significant figures).
Learn more about gravitational potential energy here:
https://brainly.com/question/3910603
#SPJ11
How do the four properties of liquids relate to the polarity of a molecule?Include the following terms in your answer: bonds, polar, non-polar, adhesion, cohesion,surface tension, capillary rise. This summary should be several sentences long. Needs to be a short summary including those words. Please HELP!
Answers
Answer
In summary
A number of properties of liquids, such as cohesion and adhesion, are influenced by the intermolecular forces within the liquid itself. Cohesion are various intermolecular forces that hold solids and liquids together while adhesion is the ability of a liquids to stick to an unlike substance. The rise or fall of a liquid in a capillary tube is governed by the balance of cohesive and adhesive forces. Lastly, surface tension is a fundamental property of the surface of liquid, it is responsible for the curvature of the surfaces of liquids dues to the polarity that exists in the liquid molecules.
Explanation
Liquids flow because the intermolecular forces between molecules are weak enough to allow the molecules to move around relative to one another. The forces are attractive when a negative charge interacts with a nearby positive charge and repulsive when the neighboring charges are the same, either both positive or both negative. Molecules are held together by polar covalent bonds – which means that the electrons are not evenly distributed between the bonded atoms. This uneven distribution in the covalent bonds of the molecules results in a partial charge.
The liquid molecules don’t interact particularly strongly with each other because the intermolecular forces are weak. The primary intermolecular forces – are London dispersion forces, which for small molecules are the weakest types of intermolecular forces. These weak forces lead to low cohesion. The molecules don’t interact strongly with each other, so they can slide right past one another.
Adhesion is the tendency of a compound to interact with another compound. (Remember that, in contrast, cohesion is the tendency of a compound to interact with itself.) Adhesion helps explain how liquids interact with their containers and with other liquids. One example of an interaction with high adhesion is that between water and glass. Both water and glass are held together by polar bonds. Therefore, the two materials can also form favorable polar interactions with each other, leading to high adhesion.
how does the name of CaS
differ from the name of CdS?
Answers
The names of the compounds differ by the type of metal present in them, which are Calcium (Ca) and Cadmium (Cd)
From the question,
We are to determine the how the name of CaS differ from the name of CdS
First, we will determine the identities of the elements in the given compounds
For CaSCa represents calcium
and
S represents sulfur
∴ The compound is named Calcium sulfide
For CdSCd represents the element Cadmium
and
S represent the element Sulfur
∴ The compound is named Cadmium sulfide
Hence, the names of the compounds differ by the type of metal present in them, which are Calcium (Ca) and Cadmium (Cd)
Learn more here: https://brainly.com/question/19078303
Hey guys, about to submit my draft for business and chemistry.
What are some things I can do to spice it up and make it better (little adjustments) that can have a big impact when the teacher is reading it?
Answers
Answer:
so u can put on hot sauce to spice it up
and give it to ur teacher
It's fruitfull to add some more information and some acquired knowledge...
Hope it is helpful !
7. The structural formula below is incomplete. It shows all of the carbon-hydrogen bonds, but none of the carbon-carbon bonds. Draw the MISSING BONDS BETWEEN THE CARBON ATOMS ONLY! (Do not add any other atoms to the structural formula below.) What is the name of the structural formula?

Answers
Step-by-step explanation:
The number of carbon atoms present in the structure is 5
Each carbon atom carry four hydrogen atoms
The above organic structure is Pentane
Having a molecular formula of C5H12
