Answers
Answer:
C) or group 16
Explanation:
Edg 2020
The element has six valence electrons available for bonding belonging to group 16 in the periodic table. Therefore, option (C) is correct.
Why does the element of the same group have the same number of valence electrons?The elements with a similar outer electronic configuration in their atoms are organized in the same group of the modern periodic table. These valence electrons decide the chemical properties and are responsible for the reactivities of the elements.
The chemical behavior of chemical elements depends on the number of valence electrons in their valence shell. Elements arranged in the same group have the same number of valence electrons. Therefore, elements placed in the same group have similar chemical properties.
The element of group 16 have a general valence electron configuration is ns²np⁴. Therefore, all elements present in group 16 have six valence electrons available for bonding with other atoms.
Learn more about elements with similar chemical behavior, here:
brainly.com/question/5364617
#SPJ6
Related Questions
For the decomposition of ammonia on a platinum surface at 856 °C
2 NH3(g)N2(g) + 3 H2(g)
the average rate of disappearance of NH3 over the time period from t = 0 s to t = 5.48×103 s is found to be 1.50×10-6 M s-1.
What is the average rate of appearance of H2 over the same time period?
M s-1.
Answers
\(\\ \tt\leadsto \dfrac{d[NH_3]}{dt}=1.50\times 10^{-6}\)
dt remains same for reaction\(\\ \tt\leadsto \dfrac{d[H_2]}{dt}=\dfrac{3}{2}\dfrac{d[NH_3]}{dt}\)
\(\\ \tt\leadsto \dfrac{d[H_2]}{dt}=\dfrac{3}{2}(1.5\times 10^{-6})\)
\(\\ \tt\leadsto \dfrac{d[H_2]}{dt}=2.25\times 10^{-6}Ms^{-1}\)
M is molarity here not metre
The average rate of appearance of H2 over the time period from t = 0 s to \(\rm t = 5.48\times10^3 s\ is\ 2.25\times10^{-6}\) M/s.
To find the average rate of appearance of \(\rm H_2\), we can use the stoichiometry of the reaction to relate it to the rate of disappearance of \(\rm NH_3\).
From the balanced equation: \(\rm 2NH_3(g) - > N_2(g) + 3 H_2(g)\)
We can see that for every 2 moles of \(\rm NH_3\) that disappear, 3 moles of \(\rm H_2\) appear.
Given that the average rate of disappearance of \(\rm NH_3\ is\ 1.50\times10^{-6} M/s\), we can calculate the average rate of appearance of \(\rm H_2\) as follows:
Average rate of appearance of \(\rm H_2\) = (3/2) x (Average rate of disappearance of \(\rm NH_3\))
Average rate of appearance of \(\rm H_2\) = (3/2) x \(\rm (1.50\times10^{-6} M/s)\)
Average rate of appearance of \(\rm H_2\) = \(\rm 2.25\times10^{-6} M/s\)
Therefore, the average rate of appearance of \(\rm H_2\) over the time period from t = 0 s to \(\rm t = 5.48\times10^3 s\ is\ 2.25\times10^{-6}\) M/s.
Know more about stoichiometry:
https://brainly.com/question/28780091
#SPJ3
A 3.5 L gas sample at 45°C and a pressure of 89.5 kPa expands to a volume of 6.50L. The final pressure of the gas is 49.5 kPa. What is the final temperature of the gas?
Answers
The concept combined gas equation is used here to determine the final temperature of the gas. These laws relate one thermodynamic variable to another holding everything else constant.
The combination of Boyle's law, Charles's law and Gay - Lussac's law give rise to the combined gas law. The state of a gas is determined by the macroscopic and microscopic parameters like pressure, volume, temperature, etc.
The combined gas equation is:
P₁V₁ /T₁ = P₂V₂ /T₂
T₂ = P₂V₂T₁ / P₁V₁
T₁ = 45 + 273 = 318 K
T₂ = 49.5 × 6.50 × 318 / 89.5 × 3.5 = 326.62 K
To know more about combined gas law, visit;
https://brainly.com/question/15805978
#SPJ1
In an isolated system, two copper bars at different temperatures transfer energy until both are at the same temperature. How would the transfer of
energy be different if the bars were in an open system?
OA Energy transfer would occur only between the copper bars.
OB. Energy transfer would occur between the copper bars and the surroundings.
OC. No energy transfer would occur between the copper bars or the surroundings.
OD. Energy transfer would occur only with the surroundings.
Answers
The manner in which the transfer of energy would be different if the bars were in an open system is as follows: Energy transfer would occur between the copper bars and the surroundings (option B).
What is law of conservation of energy?The law of conservation of energy principle stating that energy may not be created or destroyed.
An isolated system exchanges neither energy nor matter with the surroundings. According to this question, two copper bars at different temperatures transfer energy until both are at the same temperature.
However, in an open system, some of the energy would be transferred to the surroundings.
Learn more about energy transfer at: https://brainly.com/question/13087586
#SPJ1
What is the percent of C in Ca(C2H302)2? (Ca = 40.08 gkmol, C = 12.01 g/mol, H= 1.01 g/mol, O = 16.00 g/mol) [?1%C Round your answer to the hundredths place. [?] % C
Answers
Answer:
Ca(C2H3O2)2 has 30.41% carbon by volume
Explanation:
WILL GIVE 50 POINTS AND BRAINLIEST
Plate Tectonics Lab Report
Instructions: In the Plate Tectonics lab you will investigate the interactions between continental and oceanic plates at convergent, divergent, and transform boundaries around the globe. Record your observations in the lab report below. You will submit your completed report.
Name and Title:
Include your name, instructor's name, date, and name of lab.
Objective(s):
In your own words, what was the purpose of this lab?
Hypothesis:
In this section, please include the if/then statements you developed during your lab activity for each location on the map. These statements reflect your predicted outcomes for the experiment.
Location One: Select two events that you predict will be observed. If I explore two continental plates at a convergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Two: Select three events that you predict will be observed. If I explore two continental plates at a divergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Three: Select three events that you predict will be observed. If I explore two continental plates at a transform boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Four: Select two events that you predict will be observed. If I explore two oceanic plates at a convergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Five: Select three events that you predict will be observed. If I explore two oceanic plates at a divergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Six: Select two events that you predict will be observed. If I explore two oceanic plates at a transform boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Procedure:
The procedures are listed in your virtual lab. You do not need to repeat them here. Please be sure to identify the test variable (independent variable), outcome variable (dependent variable).
Reminder: Test variable = the item you are changing or manipulating; Outcome variable = the item you are measuring
Test variable (independent variable):
Outcome variable (dependent variable):
Data:
Record the data from each location below.
Location Name Boundary Type
(C=Convergent, D=Divergent, or T=Transform) Year Observed
(5, 10, or 20 million years) Geologic Events Observed
(earthquakes, faults, ocean formation, mountains, volcanoes, island chains, seafloor spreading)
Location One
Himalayas 5 Event 1-
20 Event 2-
Location Two
East Africa 5 Event 1-
10 Event 2-
20 Event 3-
Location Three
San Andreas fault zone 5 Event 1-
10 Event 2-
20 Event 3-
Location Four
Aleutian Islands 5 Event 1-
20 Event 2-
Location Five
Mid-Atlantic Ridge 5 Event 1-
10 Event 2-
20 Event 3-
Location Six
Alpine Fault 5 Event 1-
20 Event 2-
Conclusion:
Your conclusion will include a summary of the lab results and an interpretation of the results. Please write in complete sentences.
What types of geological events or changes occur at divergent plate boundaries?
What types of geological events or changes occur at convergent plate boundaries?
What types of geological events or changes occur at transform plate boundaries?
Explain how these geological processes and interactions have changed Earth's surface through the years. Be sure to use evidence to support your answer.
Answers
Answer:
here are what i have so far, im doing this right now
Explanation:
Plate Tectonics Lab Report
Instructions: In the Plate Tectonic lab you will investigate the interactions between continental and oceanic plates at convergent, divergent, and transform boundaries around the globe. Record your observations in the lab report below. Y
Objective(s):
To look at interactions between continental and oceanic plates, etc.
Hypothesis:
In this section, please include the if/then statements you developed during your lab activity for each location on the map. These statements reflect your predicted outcomes for the experiment.
Location One: Select three events that you predict will be observed. If I explore two continental plates at a convergent boundary, then I will observe:
• earthquakes
• mountains
• volcanoes
Location Two: Select three events that you predict will be observed. If explore two continental plates at a divergent boundary, then I will observe:
• ocean formation
• volcanoes
• seafloor spreading
you will submit your completed report
ps; you might want to change up the objective.
A geological event is a brief, spatially diverse, dynamic and ongoing occurrence in the history of the Earth.
What is geological event?A geological event is a brief, spatially diverse, dynamic (diachronous), and ongoing occurrence in the history of the Earth that aids in the modification of the Earth system and the production of geological strata. The concept of event stratigraphy initially came up as a way to identify, analyse, and correlate how significant physical and biological events have affected the overall stratigraphical record.
Israel's Dead Sea basin Holocene sediments contain seismic activity. This can be considered a record of a geological event, an earthquake, that altered the strata. Geological events can occur over timescales of order of magnitude, from just a few seconds through millions of years, as well as on a variety of spatial scales, from the local to the globe.
1. Volcanoes and minor earthquakes
2. Volcanoes, earthquakes and fold mountains.
3. Earthquakes and fold mountains.
4. Magma from volcanoes is filled with nutrients that makes land fertile.
Therefore, a geological event is a brief, spatially diverse, dynamic and ongoing occurrence in the history of the Earth.
To know more about geological event, here:
https://brainly.com/question/2372671
#SPJ3
How many grams of NaOH are needed to make 100. mL of solution with a concentration of 1.5 M?
Answers
To create 100 mL of solution with a concentration of 1.5 M, 6.00 grams of NaOH are required.
The amount of NaOH needed to make 100. mL of solution with a concentration of 1.5 M can be calculated using the formula:
mass = molarity x volume x molar mass
where:
molarity = 1.5 M (given)
volume = 100. mL = 0.1 L (given)
molar mass of NaOH = 40.00 g/mol (from periodic table)
Substituting the values, we get:
mass = 1.5 mol/L x 0.1 L x 40.00 g/mol
mass = 6.00 g
Therefore, 6.00 grams of NaOH are needed to make 100. mL of solution with a concentration of 1.5 M.
To know more about the Solution, here
https://brainly.com/question/14296204
#SPJ1
The absorption of 350 calories changes the temperature of a sample of water from 35°C to 62°C. What is the mass of the water?
Answers
The mass of water used is 50.0-g and the precise warmness of water (C) is 1.0 cal/g °C. those values will give you the warmth gained in calories. Q = m × C × ∆T = 50.0 g × 1.zero cal/g°C × 5.three °C = 265 cal.
answer °C = 265 cal.
Measurement of heat is finished in energy. One calorie is the quantity of energy required to raise one gram of water one diploma Celsius. To degree heat, you divide the alternate temperature of a sample of water by way of the mass of the water.
Mass is constantly regular for a body. One way to calculate mass is Mass = quantity × density. Weight is the measure of the gravitational force performing on a mass. The SI unit of mass is "kilogram".
Learn more about mass here
https://brainly.com/question/19385703
#SPJ9
Which atom would be expected to have a half-filled 6s subshell
Answers
Answer: I think the answer is Cesium (Cs)
Explanation:
A half-filled 6s subshell would be 6s^1
Let's find the temperature when the pressure is equal to zero.
There are two ways of doing this, what are they? (equation line and what else?)
What is the temperature when the pressure equals zero? What is the significance of that number?
Answers
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
What is the gas law?The gas laws can be used to describe the behavior of the ideal gases. It is pertinent to note that the ideal gas law can strictly be applied to gases that are at a high temperature and low pressure.
We can be able to find the temperature when the pressure is equal to zero either be the use of the equation line or by experiment. In that case, we would be able to obtain the point at which the pressure drops to zero.
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
Learn more about idea gas:https://brainly.com/question/4194158
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Name the following compound from the concise formula:______.
CH3CH(CH3)CHCHCH(CH3)CH2CH3
A. 2,4-dimethyl-3-heptene
B. 2,5-dimethyl-3-heptene
C. 3,5-dimethyl-3-heptene
D. 2,5-dimethyl-4-heptene
Answers
Answer:
B. 2,5-dimethyl-3-heptene
Explanation:
Answer:
B. 2,5-dimethyl-3-heptene
Explanation:
If you hear on the news that the epicenter of an earthquake is at "Union City," this means:
the earthquake started directly below Union City
the earthquake's focus was at the surface in Union City
the earthquake destroyed Union City
Answers
Answer:
B
Explanation:
how would you prepared pure water from the mixture of impure water? explain with experiment.
Answers
Explanation:
Hello, there are alot method to separate the impurities from water. One of those & best method for purification of water is Distillation.
During distillation the given apparatus are set as shown in figure. After arrangement we put the impure water in distillation flask & there are two mouth of flask one is attached to condenser & other is covered. There are two tubes in the middle of the condenser in which water comes in from one and water goes out from the other and this happens continuously. The condenser is used to convert the collected water vapours into liquid water so that it can be collected easily in recieving flask.
\( \small \sf Thanks \: for \: joining \: brainly \: community! \)

Which of Dalton's postulates is/are incorrect?
- All atoms of a given element are identical.
- Each element consists of indivisible, minute particles called atoms.
- Atoms can be neither created nor destroyed in chemical reactions.
- Atoms of different elements have different masses.
- Atoms chemically combine in definite whole-number ratios to form compounds.
Answers
Answer:
Atoms can be neither created nor destroyed in chemical reactions.
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
PLEASE HELP
Solids that do not form a crystalline structure are called.......
I don't remember what they are called
Answers
In amorphous solids the particles do not have a repeating lattice pattern. They are also called "pseudo solids." Examples of amorphous solids include glass, rubber, gels and most plastics. Hope this helps
3.88g of NaOH is required to neutralize a spill of hydrochloric acid. A 0.516M solution of NaOH is available for use.
A. Determine the number of moles needed to complete the reaction.
B. What volume of NaOH solution is needed for this reaction?
C. What volume of a stock 1,15M solution would be used to make the solution used in the reaction?
Answers
Answer:
ftb
Explanation:
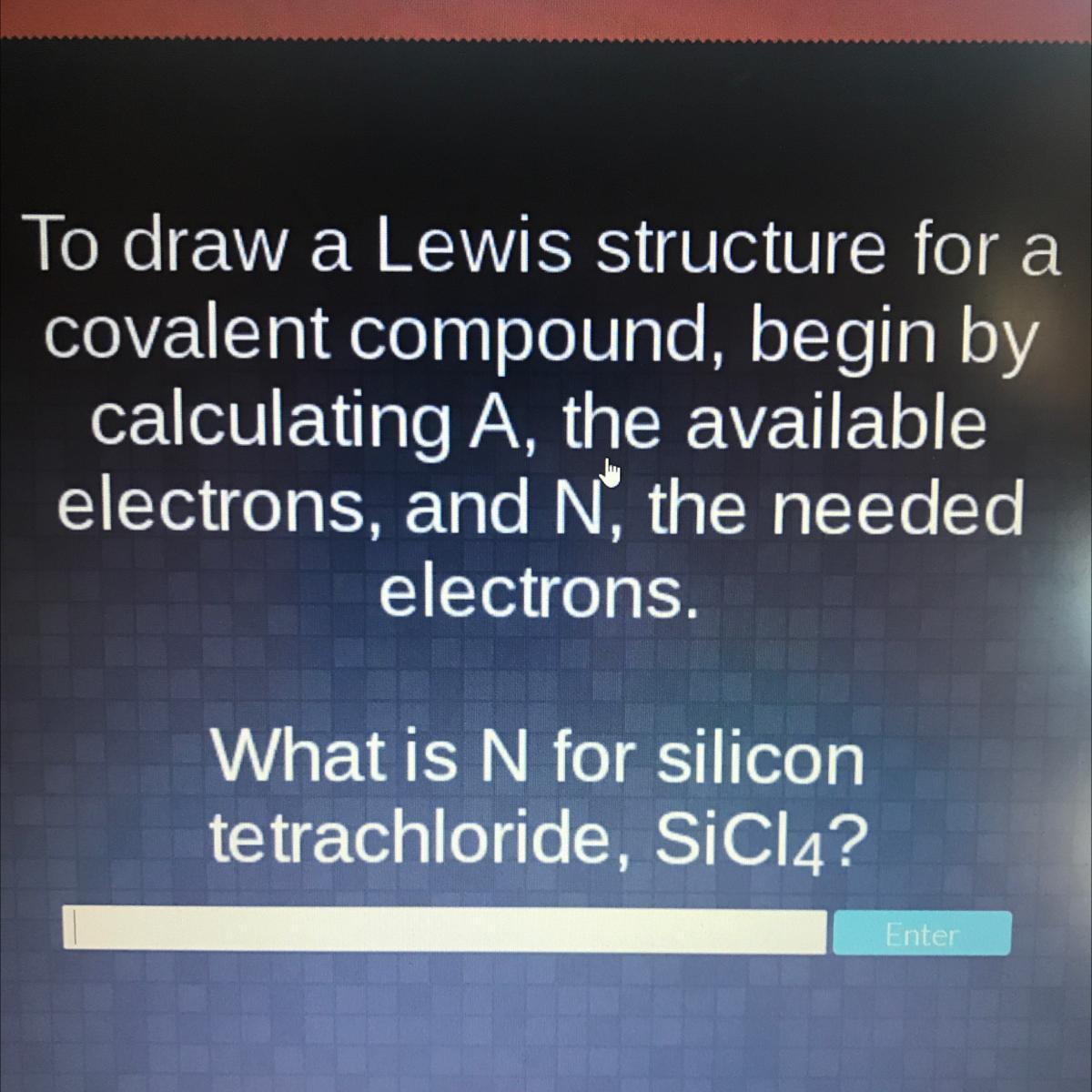
What is N for silicon tetrachloride, SiCl4?
Answers
What volume of 2.00 M NaOH must be added into the 200.0 ml of 1.00M glycolic acid solution to make a buffer solution with pH = 4.15?
Answers
We need to add 100.0 mL of 2.00 M NaOH to the 200.0 mL of 1.00 M glycolic acid solution to make a buffer solution with pH = 4.15.
What is the volume needed for the buffer solution?To make a buffer solution with pH = 4.15 using glycolic acid and sodium hydroxide, we need to calculate the amount of NaOH needed to react with the glycolic acid and form the corresponding buffer.
The pKa of glycolic acid is 3.83. Therefore, we need to use the Henderson-Hasselbalch equation to calculate the ratio of the concentrations of the conjugate base (glycolate) to the acid (glycolic acid) needed to achieve the desired pH:
pH = pKa + log([A-]/[HA])
4.15 = 3.83 + log([A-]/[HA])
0.32 = log([A-]/[HA])
10^0.32 = [A-]/[HA]
2.04 = [A-]/[HA]
Now we can use the equation for the balanced chemical reaction between glycolic acid and sodium hydroxide:
HOCH2COOH + NaOH → HOCH2COO-Na+ + H2O
We can see that one mole of glycolic acid reacts with one mole of NaOH, so we need to calculate the number of moles of glycolic acid in the 200.0 ml of 1.00M solution:
n(HA) = C(HA) x V(HA)
n(HA) = 1.00 mol/L x 0.200 L
n(HA) = 0.200 mol
Since we need a 2.04:1 ratio of [A-]/[HA], we need to calculate the number of moles of glycolate (A-) needed:
n(A-) = 2.04 x n(HA)
n(A-) = 2.04 x 0.200 mol
n(A-) = 0.408 mol
Now we can calculate the volume of 2.00 M NaOH needed to react with this amount of glycolic acid:
n(NaOH) = n(HA) = n(A-)
n(NaOH) = 0.200 mol
V(NaOH) = n(NaOH) / C(NaOH)
V(NaOH) = 0.200 mol / 2.00 mol/L
V(NaOH) = 0.100 L = 100.0 mL
Learn more about buffer solution here: https://brainly.com/question/27371101
#SPJ1
2. 4.6gof X is burnt completelyto produce 6.2g of X oxide (X,O). M (0) = 16 gmol ¹. Calculate the amount of oxygen that reacted in this experiment. [2 MARKS]
[ii] calculate the mass of 1 mole of x.[2mark]
[iii] predict and give a reason explaining the reaction of x2o in water.[1mark]
Answers
As per the given data, 1.6 grams of oxygen reacted in this experiment.
To calculate the amount of oxygen that reacted in the experiment, we need to determine the difference in the mass of X oxide (X,O) formed and the mass of X initially used.
Given:
Mass of X = 4.6 g
Mass of X oxide (X,O) = 6.2 g
To find the amount of oxygen that reacted:
Mass of oxygen = Mass of X oxide - Mass of X
= 6.2 g - 4.6 g
= 1.6 g
Therefore, 1.6 grams of oxygen reacted in this experiment.
Calculate the mass of 1 mole of X:
Given that the mass of X is 4.6 g, we can calculate the molar mass of X by dividing the mass by the number of moles:
Molar mass of X = Mass of X / Number of moles of X
Molar mass of X = 4.6 g / 0.1 mol
Molar mass of X = 46 g/mol
Therefore, the mass of 1 mole of X is 46 grams.
Thus, the answer is 46 grams.
For more details regarding moles, visit:
https://brainly.com/question/30885025
#SPJ1
Describe the orbital notation in detail. For example, 1s: up arrow down arrow; 2s up arrow down arrow; 2p three up arrows for potassium.
1s2 2s2 2p6 3s2 3p6 4s1
Answers
Orbital notation is a way of representing the electronic configuration of an atom, which describes the arrangement of electrons in its various energy levels or orbitals.
How is each orbital is represented by in the orbital notation?In this notation, each orbital is represented by a box or circle, and the electrons are represented by up or down arrows, which indicate their spin. The number and arrangement of boxes and arrows in the notation follow the rules of the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
The Aufbau principle tells that electrons fill the least energy orbitals before filling higher energy orbitals. The first shell of an atom contains one s orbital, which can hold up to two electrons. The s orbital is represented by a single box or circle, and each electron is represented by an up or down arrow.
The electronic configuration for potassium (K) is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. In orbital notation, this would be represented as 1s: up arrow, down arrow; 2s: up arrow, down arrow; 2p: up arrow, up arrow, up arrow; 3s: up arrow, down arrow; 3p: up arrow, up arrow, up arrow; 4s: up arrow.
Learn more about orbital notation here:
https://brainly.com/question/1156391
#SPJ1
Balance the following equation:

Answers
Answer:
\(C_{5} H_{12} + 8O_{2} \\\ - > 5CO_{2} + 6H_{2}O\)
Explanation:
Blank 1: remain blank or put 1
Blank 2: 8
Blank 3: 5
Blank 4: 6
Purpose is to have the same amount of each element on both side of equation
To calculate the atoms of an element in a given molecule, we need to multiply stoichiometry by the number that is written on the foot of that element. The balanced equation is
C\(_5\)H\(_12\)+O\(_2\)\(\rightarrow\)5CO\(_2\)+6H\(_2\)O
What is Balanced equation?Balanced equation is the one in which the total number of atoms of a species on reactant side is equal to the total number of atoms on product side.
The unbalanced equation is
C\(_5\)H\(_12\)+O\(_2\)\(\rightarrow\)CO\(_2\)+H\(_2\)O
The number of atoms of carbon on reactant side is 5 and on product side it is 1. so to balance it we need to multiply CO\(_2\) by 5.
C\(_5\)H\(_12\)+O\(_2\)\(\rightarrow\)5CO\(_2\)+H\(_2\)O
The hydrogen atom on reactant side is 12 while on product side it is 2, so multiply H\(_2\)O by 6.
C\(_5\)H\(_12\)+O\(_2\)\(\rightarrow\)5CO\(_2\)+6H\(_2\)O
To multiply oxygen we need to multiply O\(_2\) by 8.
C\(_5\)H\(_12\)+8O\(_2\)\(\rightarrow\)5CO\(_2\)+6H\(_2\)O
Therefore, the balanced equation is
C\(_5\)H\(_12\)+O\(_2\)\(\rightarrow\)5CO\(_2\)+6H\(_2\)O
Learn more about the balanced equation, here:
https://brainly.com/question/7181548
#SPJ1
Step 7: Put the Metal in the Water and Measure Temperature Changes (Copper)
Answers
When copper is placed in water, it reacts with the water molecules to form copper(II) ions and hydrogen gas. The reaction is exothermic, which means it releases heat energy into the surroundings. By measuring the temperature changes that occur, we can determine the amount of heat that is released by the reaction.
The temperature changes can be measured using a thermometer. We can place the copper metal in a container of water and take the initial temperature reading. Then, we can add the copper to the water and record the temperature change over time. By monitoring the temperature changes, we can observe the exothermic reaction taking place.
The heat released by the reaction between copper and water has many practical applications, including in the design of power plants and in the production of steam for heating and electricity generation. Therefore, understanding the heat released during this reaction is important for a variety of scientific and engineering fields.
In conclusion, step 7 of putting copper metal in water and measuring the temperature changes allows us to observe and measure the heat released by the exothermic reaction between copper and water, which has important applications in various scientific and engineering fields.
Know more about exothermic here:
https://brainly.com/question/2924714
#SPJ11
Answer:
Aluminum
100 C22.4 C27.1 C4.7 C72.9 Ccopper
100 C22.7 C24.6 C1.9 C75.4 CIron
100 C22.5 C24.9 C2.4 C75.1 CLead
100 C22.6 C23.3 C0.7 C76.7 CThe Final Slide:
Aluminum- 0.90
Copper- 0.35
Iron- 0.44
Lead- 0.12
Explanation:
I hope this helps! :))))
A chemical equilibrium exists when: A chemical equilibrium exists when: there are equal amounts of reactants and products. the rate at which reactants form products is the same as the rate at which products form reactants. the sum of reactant and product concentrations equals one mole. reactants are completely changed to products. the rate at which reactants form products becomes zero.
Answers
Answer: A chemical equilibrium exists when the rate at which reactants form products is the same as the rate at which products form reactants.
Explanation:
When the concentration of both the reactants and products do not change with time then it means the chemical reaction has reached to a state of chemical equilibrium.
For example, \(CO(g) + Cl_{2}(g) \rightleftharpoons COCl_{2}(g)\)
Therefore, we can conclude that a chemical equilibrium exists when the rate at which reactants form products is the same as the rate at which products form reactants.
Hydrogen sulfide gas is a flammable gas. The gas burns with
a blue flame according to the following equation:
2 H₂S (g) + 3 O2 (g) => 2 SO2 (g) + 2 H₂O(g)
5.00 L of hydrogen sulfide gas and 7.00 L of oxygen were
allowed to react.
Calculate the theoretical yield, in L (liters), of sulfur dioxide.
All the gases were at the same temperature and pressure.

Answers
Answer:
4.67 liters of SO2 with sig figs
Explanation:
To do this reaction, we first must find the limiting agent.
in this problem, 5 liters of H2S are reacted with 7 liters of O2, and each two moles of H2S corresponds to 3 moles of O2. Assuming ideal behavior, 1 liter of H2S has the same number of moles as 1 liter of O2 has. So, 2 liters of H2S react with 3 liters of O2. To find the limiting reagent, focus on one reagent and calculate how much of the other reagents would be needed to fully consume that one reagent.
Focus on H2S:
5 liters of H2S would require 7.5 liters of O2 (since each 2 liters of H2S requires 3 liters of O2)
Focus on O2:
7 liters of O2 would require 14/3, or 4.666... liters of H2S.
Since we don't have 7.5 liters of O2 (we only have 7 liters) to react with all 5 liters of H2S, we would say that O2 is the limiting reagent, and that we have 7 liters of it.
Next, figure out how much product can be produced per mole of that limiting reagent. 3 moles of O2 and 2 moles of H2S turns into 2 moles of SO2 and 2 moles of H2O. Thus, the ratio of O2 to SO2 is 3:2
We have 7 liters of O2, and since the ratio of O2 to SO2 is 3:2, we can set up the equation:
7:x = 3:2 where x is the number of liters of SO2
cross multiply (or solve with another method) to get:
x = 14/3, or 4.666... liters of SO2
Is the formula of both copper (1) superoxide and copper (2) peroxide CuO2?
Answers
Answer:
Copper(2+) is an ion of copper carrying a double positive charge. It has a role as a cofactor. It is a divalent metal cation, a copper cation and a monoatomic dication. Oxidation means loss of electron and reduction means gain of electron. In this case Cu donates 2 electrons to form Cu^2+ ion,therefore,its an oxidation process.
Explanation:
Which of the following atoms are stable?
Lithium
Aluminum
Carbon
Neon
Answers
Answer:
Neon
Explanation: The valence electrons of Neon are filled.
Hope it helps you:)))))
Have a good day.
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
What do you think is the strongest chemical bonding?why? OWN PERSPECTIVE PLS!!
Answers
explanation: answer on safari hope this helps!