an a Use the You need to make ar solid barium sulfide should you add?
Answers
To make solid barium sulfide, you would need to react barium metal with elemental sulfur. The balanced chemical equation for this reaction is:
Ba(s) + S(s) → BaS(s)
To carry out this reaction, you would need to add excess sulfur to the barium metal. This ensures that all the barium is consumed in the reaction, and no excess barium remains. The excess sulfur can be removed by washing the product with a suitable solvent.
It is important to note that the reaction between barium and sulfur can be exothermic, releasing heat and potentially causing a fire or explosion. Therefore, appropriate safety precautions, such as wearing gloves and eye protection and working in a well-ventilated area, should be taken when carrying out this reaction.
Learn more about barium sulfide,
https://brainly.com/question/18402799
#SPJ4
To make a solid barium sulfide (BaS) you would need to add sulfur (S) to barium (Ba) in a stoichiometric ratio of 1:1. This means that for every one mole of barium, you would need one mole of sulfur.
The reaction can be represented by the following chemical equation:
Ba + S → BaS
To carry out this reaction, you could start with a sample of metallic barium and add elemental sulfur powder to it, in a ratio of 1:1 by mole. The reaction between the two elements will produce solid barium sulfide.
It is important to note that this reaction can be highly exothermic, so appropriate safety precautions should be taken. Additionally, barium sulfide is a toxic and reactive compound, and should be handled with care.
For more question on barium sulfide click on
https://brainly.com/question/31013085
#SPJ11
Related Questions
A river with 25ppm phosphate and an upstream flow of 40 m ^3/s receives an agricultural discharge of 2.5 m^ 3 /s carrying 1000ppm phosphate. The chemical in the agricultural stream mix instantaneously with the main river flow. The phosphate has a first-order decay rate of 0.15/ day and the river has a cross sectional area of 20 m ^2
perpendicular to the direction of flow. A municipality located 90 km downstream of the agricultural stream discharge point withdraws water for municipal water supply purpose. a. Draw a schematic diagram of the control volume. b. Find the steady-state phosphate concentration in the water withdrawn 90 km downstream? c. Find the treatment requirement (\% removal) in the agricultural waste discharge to achieve 50mg/L concentration in the withdrawal location 80 km downstream? (Hint: Find the concentration of the waste-stream that will produce 50mg/L downstream concentration. Find \% removal from the difference of the influent wastewater concentration with respect to the initial waste-stream concentration, i.e., 1000mg/L )
Answers
The treatment requirement (% removal) in the agricultural waste discharge to achieve 50mg/L concentration in the withdrawal location 80 km downstream is 99.57%
a. Control Volume
The schematic diagram of the control volume is given below.
b. Steady-state Phosphate concentration in water withdrawn 90 km downstream
The steady-state phosphate concentration in the water withdrawn 90 km downstream is given by:
C2 = (Q1C1 + Q2C2)/(Q1 + Q2)
Where,
C2 = Concentration of phosphate in the water withdrawn 90 km downstream
C1 = Concentration of phosphate in the upstream water (25 ppm)Q1 = Upstream flow (40 m 3/s)Q2 = Agricultural discharge (2.5 m^3/s)C2 = ((40 x 25) + (2.5 x 1000)) / (40 + 2.5)C2 = 59.3 ppm
Therefore, the steady-state phosphate concentration in the water withdrawn 90 km downstream is 59.3 ppm.c. Treatment requirement (% removal) in the agricultural waste discharge to achieve 50mg/L concentration in the withdrawal location 80 km downstream
The concentration of the waste-stream that will produce 50mg/L downstream concentration is given by:
50 = (Q1C1 + Q2C2)/(Q1 + Q2)C2 = ((40 x 25) + (2.5 x C2))/(40 + 2.5)50 = (1000 x 2.5) / (40 + 2.5) + (40 x 25) / (40 + 2.5)C2 = 4.3 ppm
The % removal from the difference of the influent wastewater concentration with respect to the initial waste-stream concentration is given by:
% removal = (C in - C out) / C in x 100Where,Cin = Influent wastewater concentration (1000 ppm)
C out = Concentration of waste-stream required to produce 50 ppm downstream concentration (4.3 ppm)\% removal = (1000 - 4.3) / 1000 x 100\% removal = 99.57%
Therefore, the treatment requirement ( % removal) in the agricultural waste discharge to achieve 50mg/L concentration in the withdrawal location 80 km downstream is 99.57%.
To know more about waste discharge visit:
https://brainly.com/question/10156015
#SPJ11
Mayo clinic suggests up the maximum amount of caffeine a healthy adult should drin in a day is equivalent to four cups of brewed coffee, 10 cand of cola or two "energy shot" drinks. If you were to drink 4 cups of that coffee that would be 32 oz of coffee. If on did the math that would be a volume of 0.956 liters of Coffee.(The density of the Caffeine is
1.23g/cm^3 and the Chemical formula is C8H10N4O2)
How much does the coffee weigh in grams?
How many moles are in the coffee?
How many Atoms (aka molecules) of coffee are there?
Answers
The mass of coffe is 1176 g. The number of moles of caffiene is 6 moles and the number of caffiene molecules is 3.6 * 10^24 molecules
What is coffe?Coffe is an energy drink that is commonly made of caffiene. It is the active ingridient in coffe.
Now we have;
Volume of the coffe = 0.956 liters or 956 cm^3
density of the coffe = 1.23g/cm^3
Mass of coffe = 1.23g/cm^3 * 956 cm^3 = 1176 g
Number of moles of caffiene= mass /molar mass = 1176 g/194 g/mol = 6 moles
Now;
If 1 mole contains 6.02 * 10^23 molecules
6 moles contains 6 moles * 6.02 * 10^23 molecules/1 mole
= 3.6 * 10^24 molecules
Learn more about molecules : https://brainly.com/question/19922822
In the formula XS04, the symbol X could represent the element*
O (1) AI
O (2) Ar
O (3) Mg
0 (4) Na
Answers
Why does alcohol evaporate faster than water? *
1.Alcohol evaporates faster than water because alcohol molecules are hotter
2.Alcohol evaporates faster than water because alcohol molecules are not as attracted
to each other as water molecules are
3.Alcohol evaporates faster than water because alcohol molecules are more attracted
to each other than water molecules are (Which one I need help)
Answers
Answer:
Number oneExplanation:
Alcohol has a lower boiling point than water. Water boils at 100 degrees Celsius (212 degrees Fahrenheit), while Alcohol boils at 82 degrees Celsius (179.6 degrees Fahrenheit).Astatine-210 has a half-life of 8.08 days. What fraction of a sample of astatine-210 is left unchanged after 16.16 days?
Answers
Answer:
0
Explanation:
Given parameters:
Half-life = 8.08days
Unknown:
What fraction is left unchanged after 16.16days = ?
Solution:
The half - life of a substance is the time taken for the half of a radioactive material to decay to half.
Day 0 Day 8.08 Day 16.16
100% 50% 0% Parent
0% 50% 100% Daughter
After 16.16 days, non of the original sample will remain unchanged.
if the specific gravity of the mixture is 0.781, what are the w/v concentrations of lactic acid, salicylic acid, and trichloroacetic acid in the mixture?
Answers
What are the relative w/v concentrations of lactic acid, citric acid, and boric acid if the mixture's specific gravity is 0.781? What are the % weight-to-volume conversions if the mixture's specific gravity is 0.781?
(Density of the solution / Water Density)
density of mixture = (specific gravity of mixed * density of waters) = (0.781 * 1) = 0.781 g/mL density = (mass/volume) specific gravity = 0.781
Lactic acid concentration in the mixture = (mass of latic acid / quantity of mixture) *100 = (4/128.04)100 = 3.12%. Volume of mixture = (mass / density) = (100 /0.781) =128.04 mL.
What level of fluoride ion should be present in drinking water?
Detailed Solution: 1 mg/l
1.50 mg per liter is the proper answer. The World Health Assembly advised that the maximum allowed level of fluoride present in drinking water for a number of nations, including Canada, China, India, Argentina, and the European Union, be 1.5 mg/l. A fluoride level of 1 mg/l is ideal for drinking water.
To know more acid about:
https://brainly.com/question/14072179
SPJ4
The w/v levels of lactic acid, benzoyl peroxide, plus trichloroacetic acid as in combination are 1.56% if the mixture has a specific gravity of 0.781.
What level of fluoride ion should be present in drinking water?1 mg/l 1.50 mg per liter is the proper answer. The World Health Assembly advised that the maximum allowed level of fluoride present in drinking water for a number of nations, including Canada, China, India, Argentina, and the European Union, be 1.5 mg/l. A fluoride level of 1 mg/l is ideal for drinking water.
(Density of solution / Water Density) = Specific Gravity
density of mixture = (specific gravity of mixed * densities of water) = (0.781 * 1) = 0.781 g/mL density = (mass/volume) specific gravity = 0.781
Volume of the mixture = (Mass of Lactic Acid / Volume of the mixture) = (100 /0.781) =128.04 mL w/v Amount of Lactic in the mixture Salicylic acid concentration in the mixture is *100 = (4/128.04)100 = 3.12% w/v = (mass of Salicylic / volume of mixture). Trichloroacetic acid concentration in the combination: *100 =(5/128.04)100 = 3.9% w/v (i.e., (mass of Trichloroacetic acid / volume of mixture) *100 =(2/128.04)100 = 1.56%
To know more acid about:
brainly.com/question/14072179
#SPJ4
Create a model using images that would show what would happen to the igneous rock when it is exposed to different energy sources.
Answers
igneous is moved to Earth's surface and exposed to energy from the sun, it could weather into smaller rock pieces that could form sedimentary rock

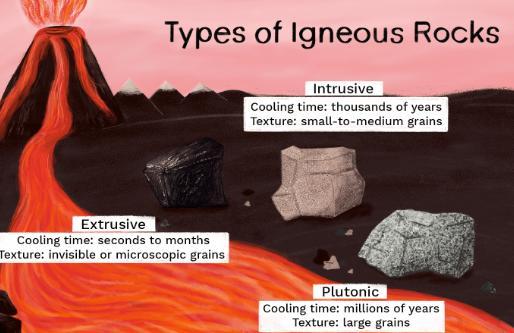
Some igneous rock forms from magma that cools slowly within Earth’s crust. Briefly describe the most likely crystallization and texture of the rock.
Answers
some assumptions from the kinetic molecular theory are listed below. which one is most frequently cited to explain diffusion of a gas?
Answers
Some assumptions from the kinetic molecular theory , one is most frequently cited to explain diffusion of a gas is the gas consist of tiny particles moving in the random straight line motion.
The assumption of the kinetic molecular theory are :
the gases are made up of tiny particles and these tiny particles are in random motion.there is no force of attraction between the gas particles.the temperature of the gas id depend on the kinetic energy og the gas particles.Thus, the gas is the gas consist of tiny particles moving in the random straight line motion explain the diffusion of a gas.
To learn more about kinetic molecular theory here
https://brainly.com/question/5954988
#SPJ4
A suspension of yeast supplied with glucose as its source of energy was transferred from an aerobic environment to an anaerobic one. Which result would you predict for the rate of glucose utilization and the rate of ethanol production after the transfer? assume that the yeast must expend the same amount of energy to survive in both environments.
Answers
After the transfer from aerobic to anaerobic environment the rate of ethanol production will increase and the rate of glucose utilization would decrease.
Why the rate of ethanol production increases and glucose is broken down?See when the yeast was pacing in aerobic environment that is in the presence of oxygen it does not grow with fast speed.When yeast is transferred to anaerobic environment it grows rapidly.In anaerobic environment yeast breaks down glucose.By breaking down glucose releases ethanol and an appreciable amount of CO2.That's why yeast grows rapidly in anaerobic environment comparatively.Expending the same amount of energy yeast survives the best in anaerobic environment.To know more about yeast visit:
https://brainly.com/question/11600258
#SPJ4
1. You may be using medium for shoot regeneration from leaf explants of a plant in Expt-5. The plant media may contain the plant growth regulators (hoones) BA and NAA. The molecular weight of BK is 72 A : and NAA is 186. The media is pH to 5.8. (a) Before making the plant media, you found the pH to be 3.6. What would you add quiekly to get it to a pH of 5.8 (give a specific name of the solution)? Why? (1 pt) (b) How much BA will be weighed fot a 1M solution? (Y po) (c) Convert your answer from (b) to mg/ml. (Y/ pt) (d) Convert your answer from (c) to mg 1 . (1 pt) (e) How much BA will be weighed for a 5mM solution? (1/4pt) (f) Convert your answer from (c) to mg/ml. ( /4pt ) (g) Convert your answer from (f) to mg/L. (H/ pt) (h) Your stock solution of BA is 5mM and your working solution is 0.2mg/.. What volume of the stoc be added to 250ml of medium? [Hint: fook at the previous answers Keep to 4 decimal pts.) (3 pts Convert your answer from (h) to μI, and which pipettor will you use to aliquot the B. A? (1 pt)
Answers
(a) To get the pH of the media to 5.8, you would add NaOH solution. NaOH is used as a basic solution, and when it is added to a solution, it will increase the pH of the solution.
(b) The molecular weight of BA is 225.3. To prepare a 1M solution, you would have to weigh out 225.3 grams of BA.(c) To convert a 1M solution of BA to mg/mL, you can use the following equation: 1 mole = molecular weight in grams; 1000 millimoles = 1 mole. So, 1 M = 1000 mg/mL. Therefore, a 1M solution of BA is equivalent to 1000 mg/mL .(d) To convert a concentration of 1000 mg/mL .
Therefore, to calculate the weight required for a 5 mM solution, use the following formula :Mass of BA = molarity × volume × molecular weight= 5 × 0.001 × 225.3= 1.1265 grams(f) To convert a concentration of 5 mM to mg/mL, we use the following formula: Concentration (mg/mL) = (Concentration (mM) × Molecular weight) / 1000= (5 × 225.3) / 1000= 1.1265 mg/mL(g)
To convert a concentration of 1.1265 mg/mL to mg/L, we multiply by 1000, so 1.1265 mg/mL = 1126.5 mg/L.(h) Given that the stock solution of BA is 5 mM and the working solution is 0.2 mg/mL.
To know more about increase visit:
brainly.com/question/19383315
#SPJ11
The first excited vibrational energy level of diatomic chlorine (Cl2) is 558 cm−1 above the ground state. Wavenumbers, the units in which vibrational frequencies are usually recorded, are effectively units of energy, with 1 cm−1=1.986445∗10−23 J. A. If every vibrational energy level is equally spaced, and has a degeneracy g, of 1 , sum over the lowest 4 vibrational levels to obtain a vibrational partition function Q, for chlorine at 25∘C. Your answers will be as sum of exponentials, simplify them as much as you can. B. Let the N1 and N2 be the population of chlorine molecules in the first and second excited vibrational energy levels respectively. Find the relative population between the excited states N1N2, at 298 K(25∘C) [Convert energy into Joules first before finding the exponentials for the partition function. See practice problem set 5 . The ground state is at 0 J energy level. The Boltzmann constant, kB= 1.38065×10−23 J]
Answers
The vibrational partition function (Q) for chlorine at 25°C, summing over the lowest 4 vibrational levels, is given by the simplified expression: Q = e^(-2.220).
The relative population between the first and second excited vibrational energy levels (N1/N2) of chlorine at 298 K (25°C) can be calculated using the relative Boltzmann factors, taking into account the energies of the levels and the Boltzmann constant.
The vibrational partition function (Q) represents the sum of the Boltzmann factors for all the vibrational energy levels. For a diatomic molecule like chlorine (Cl2), assuming equally spaced vibrational energy levels and a degeneracy (g) of 1 for each level, we can calculate the partition function.
To calculate Q, we sum over the lowest 4 vibrational levels, taking into account the energy spacing between levels.
The energy spacing between levels is given as 558 cm^(-1), which we convert to Joules using the conversion factor of 1 cm^(-1) = 1.986445 × 10^(-23) J.
Using the formula for the partition function:
Q = e^(-E1/(kT)) + e^(-E2/(kT)) + e^(-E3/(kT)) + e^(-E4/(kT))
Substituting the values:
Q = e^(-5581.98644510^(-23)/(1.3806510^(-23)(25+273)))
Simplifying the exponent and performing the calculations:
Q ≈ e^(-2.220)
Therefore, the vibrational partition function (Q) for chlorine at 25°C, summing over the lowest 4 vibrational levels, is approximately e^(-2.220).
The relative population between the first and second excited vibrational energy levels (N1/N2) of chlorine at 298 K (25°C) can be calculated using the relative Boltzmann factors, taking into account the energies of the levels and the Boltzmann constant.
The relative population (N1/N2) between two vibrational energy levels can be determined using the Boltzmann factors, which depend on the energies of the levels and the temperature.
The energy difference between the ground state and the first excited level is given as 558 cm^(-1), which we convert to Joules using the conversion factor of 1 cm^(-1) = 1.986445 × 10^(-23) J.
Using the Boltzmann factor formula:
N1/N2 = e^(-ΔE/(k*T))
Substituting the values:
N1/N2 = e^(-5581.98644510^(-23)/(1.38065*10^(-23)*298))
Simplifying the exponent and performing the calculations:
N1/N2 ≈ e^(-1.524)
Therefore, the relative population between the first and second excited vibrational energy levels (N1/N2) of chlorine at 298 K (25°C) is approximately e^(-1.524).
Note: The relative population is given as a ratio of the populations between the two levels.
Learn more about Boltzmann factors here: brainly.com/question/30763196
#SPJ11
Describe two ways the material in this lesson illustrates the importance of creativity in scientific investigation
Answers
Answer:
Dalton used creativity to modify Proust's experiment and interpret the results. Millikan used creativity to develop a way to measure the charge on an electron. Thomson used creativity to interpret the results of the cathode ray tube experiment.
Explanation:
Creativity enables scientists to devise new ways of solving old problems.
I do not know what your material contains, however, creativity is key in scientific investigation. The term "science" is coined from the Latin word "scientia" which means knowledge.
In science, we endeavor to create new knowledge through research and innovation. This requires that a scientist must think of new and efficient ways of solving old problems. This underscores the centrality of creativity in scientific investigation.
Learn more: https://brainly.com/question/12108425
what do the abbreviations such as phe, ile, ala, and gly in model 1 represent?
Answers
Phe, Ile, Ala, and Gly are the abbreviations for the four amino acids phenylalanine, isoleucine, alanine, and glycine respectively. They are four of the twenty protein-forming amino acids that are commonly found in proteins and are used in the formation of peptide bonds.
What is amino acids?Amino acids are organic compounds that are composed of amine (-NH2) and carboxylic acid (-COOH) functional groups, along with a side chain (R group) specific to each amino acid. The precise order of amino acids in a protein is determined by the genetic code and determines the structure and function of the protein. There are twenty different amino acids that are commonly found in proteins and are essential for life. They play a key role in many biological processes, including metabolism and cellular signaling. Amino acids are also important components of enzymes and can be used as a source of energy.
To learn more about amino acids
https://brainly.com/question/24106148
#SPJ4
Help! I am in a hurry! Will get brainliest if correct!

Answers
Explain how to convert a mass of compound A to a mass of compound D in words using the following reaction:
2A + 4B = C + 3D
You don't have to just give me the answer but if you can help me understand id appreciate it greatly
Answers
Answer:
1. Convert the mass of A to moles dividing in molar mass
2. Convert the moles of A to moles of D (2mol A = 3mol D)
3. Convert the moles of D to mass multiplying by its molar mass
Explanation:
As you can see in the equation, 2moles of A produce 3 moles of D, the equation is:
2mol A = 3mol D
But in the problem you are having the mass of A and you need the mass of D. That means you require to convert the mass of A to moles. With the moles of A you can find the moles of D and, as last, you need to convert the moles of A to mass.
To convert mass to moles or vice versa you must use Molar Mass. That means, the answer is:
1. Convert the mass of A to moles dividing in molar mass2. Convert the moles of A to moles of D (2mol A = 3mol D)3. Convert the moles of D to mass multiplying by its molar massLithium and bromine react to form lithium bromide. How many moles of bromine must be used to produce 5.5 moles of lithium bromide?
Answers
ANSWER
EXPLANATION
Given that
The number of moles lithium bromide is 5.5 moles
Firstly, write a balanced equation for the reaction
\(\text{ 2Li + Br}_2\rightarrow\text{ 2LiBr}\)Find the number of moles of bromine using stoichiometry ratio
Let x represents the number of bromine
\(undefined\)what substances have an arrhenius base?
Answers
Potassium hydroxide-mg(oh)2
lactic acid, a chemical responsible for muscle fatigue, is a monoprotic acid
Answers
Yes Lactic acid is a monotropic acid.
Lactic acid, a chemical responsible for muscle fatigue, is a monoprotic acid.
A monoprotic acid is a kind of acid that can donate only one hydrogen ion per molecule to an aqueous solution. In the case of lactic acid, it has only one hydrogen ion to donate. Therefore, it is a monoprotic acid.
Lactic acid is the chemical responsible for muscle fatigue. When the body performs anaerobic exercises such as sprinting or weight lifting, it rapidly produces ATP without sufficient oxygen and leads to lactic acid release leading to muscle pain. This the reason for muscle pain after heavy exercises.
To know more about Monotropic acid visit:
https://brainly.com/question/29068526
#SPJ11
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
H3PO4(aq) = H+ (aq) + H2P04- (aq)
What happens if NaOH is added?
А
B
shifts to
reactants
remains
the same
shifts to
products
Answers
When NaOH is added to the buffer that is prepared by adding NaH2PO4 to a solution of H3PO4, the reaction equilibrium shifts towards products.
Weak buffer solutions are used to counteract excess acid or base in a solution such that the pH is unaffected. Weak acid or base and the suitable acid or base salt are used to make it.
Strong base sodium hydroxide (NaOH) splits into sodium and hydroxyl ions when it is heated. There is an increase in hydroxyl ions when it is added to a buffer solution. Because buffer contains weak acid (HA), the acid donates its proton so that the hydroxyl ions of NaOH react with this proton to form water and its conjugate pair, eventually canceling out their effect in solution. Following are the responses:
NaOH >> Na(OH)+ + OH
The extra ions are therefore neutralized.
For more information on buffer solution kindly visit to
https://brainly.com/question/24262133
#SPJ4
which buffer solution will have the greatest buffer capacity? group of answer choices a solution that is 0.1 m in both ammonia and ammonium nitrate a solution that is 1.0 m in hbro and 0.1 m in nabro. a solution that is 1.0 m in both acetic acid and sodium acetate a solution that is 0.1 m in both acetic acid and sodium acetate a solution that is 0.1 m in ammonia and 1.0 m in ammonium nitrate g
Answers
The buffer solution that will have the greatest buffer capacity is the solution that is 1.0 m in both acetic acid and sodium acetate.
Buffer capacity refers to the effectiveness of a buffer in resisting changes in pH when exposed to additional acid or base. The higher the buffer capacity, the more resistant the solution is to changes in pH. The buffer solution that will have the greatest buffer capacity is the solution that is 1.0 m in both acetic acid and sodium acetate. Acetic acid is an organic acid that reacts with a strong base like sodium hydroxide to produce a salt of acetic acid, known as sodium acetate. In this reaction, one hydrogen ion of acetic acid is replaced by a sodium ion.
The chemical equation is as follows:
C2H4O2(aq) + NaOH(aq) → NaC2H3O2(aq) + H2O(l)
This reaction produces a buffer solution of acetic acid and sodium acetate. The solution has a pH of around 4.8 and acts as a buffer because the acetate ions can react with additional hydrogen ions, while acetic acid can react with additional hydroxide ions, thus preventing significant changes in pH. The other options contain the following: a solution that is 0.1 m in both ammonia and ammonium nitrate, a solution that is 1.0 m in HBrO and 0.1 m in NaBrO, a solution that is 0.1 m in both acetic acid and sodium acetate, a solution that is 0.1 m in ammonia and 1.0 m in ammonium nitrate.
Learn more about Buffer at https://brainly.com/question/22821585
#SPJ11
What is the hydronium ion concentration of a 0.400 M acetic acid solution with Ka = 1.8 × 10^-5? The equation for the dissociation of acetic acid is:
CH3CO2H(aq) + H2O (l) <-> H3O ^+ (aq) + CH3Co2^- (aq)
A) 2.7 × 10^-2 M
B) 4.2 × 10^-2 M
C) 2.7 × 10^-3 M
D) 4.2 × 10^-3 M
Answers
To find the hydronium ion concentration of a 0.400 M acetic acid solution with Ka = 1.8 × 10^-5, we can follow these steps:
1. Write the equilibrium expression for the dissociation of acetic acid:
Ka = [H3O+][CH3CO2-]/[CH3CO2H]
2. Set up an ICE table (Initial, Change, Equilibrium) for the concentrations:
CH3CO2H H3O+ CH3CO2-
Initial: 0.400 M 0 M 0 M
Change: -x +x +x
Equilibrium: 0.400-x x x
3. Substitute the equilibrium concentrations into the equilibrium expression:
Ka = (x)(x)/(0.400-x)
4. Substitute the given Ka value (1.8 × 10^-5) and solve for x:
1.8 × 10^-5 = (x)(x)/(0.400-x)
Since Ka is small, we can assume x is much smaller than 0.400, so 0.400-x ≈ 0.400:
1.8 × 10^-5 = x^2/0.400
5. Solve for x, which represents the concentration of H3O+ ions:
x^2 = (1.8 × 10^-5)(0.400)
x^2 = 7.2 × 10^-6
x = √(7.2 × 10^-6)
x ≈ 2.68 × 10^-3 M
So, the hydronium ion concentration of the 0.400 M acetic acid solution is approximately 2.7 × 10^-3 M, which corresponds to option C.
To know more about the concentration of H3O+ ions:
https://brainly.com/question/30459751
#SPJ11
Finely ground nickel (II) hydroxide is placed in a beaker of water. It sinks to the bottom of the beaker and remains unchanged. An aqueous solution of hydrochloric acid is then added to the beaker and the Ni(OH)2 disappears. Which reaction best describes what occurred in the beaker?
Your answer:
Ni(OH)2 (s) + HCl (aq) → NiO (aq) + H2 (g) + HCl (aq)
Ni(OH)2 (s) + 2 HCl (aq) → NiCl2 (aq) + 2 H2O (l)
Ni(OH)2 (s) + 2 H2O (l) → NiCl2 (aq) + 2 H2O (l)
Ni(OH)2 (s) + 2 H2O (l) → NiCl2 (aq) + 3 H2O (l) + O2 (g)
Answers
Explanation:
Acid-base reaction will give salt and water.
The 2nd option is correct.
The chemical equation Ni(OH)₂ (s) + 2 HCl (aq) → NiCl₂(aq) + 2 H₂O (l) represents what happened in the beaker as acid and base reaction gives salt and water.
What is chemical equation?
Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ2
Strontium-90 has a half-life of 28 years. If a 8.00 mg sample were stored for 112 years, what mass of 90Sr would remain?
Answers
The mass of Strontium-90 left after 112 years with an half-life of 28 years is 0.5 mg.
What is mass?Mass can be defined as the quantity of matter a body contains.
To calculate the mass of Strontium-90 left, we use the formula below.
Formula:
R' = R/(\(2^{n/t}\))............... Equation 1Where:
R' = Mass of Strontium-90 leftR = Original mass of Strontium-90n = Total time t = Half-life of Strontium-90From the question,
Given:
R = 8.00 mgt = 28 yearsn = 112 yearsSubstitute these values into equation 1
R = 8/(\(2^{112/28}\))R = 8/2⁴R = 8/16R = 0.5 mgHence, the mass of Strontium-90 left is 0.5 mg.
Learn more about mass here: https://brainly.com/question/19385703
#SPJ1
Write a balanced equation using the correct formulas and include conditions (s, l, g or aq) for each of the following reactions. Express your answer as a chemical equation. Identify all of the phases in your answer. A.) Sodium metal reacts with liquid water to form hydrogen gas and aqueous sodium hydroxide. B.) Solid phosphorus reacts with chlorine gas to form solid phosphorus pentachloride. C.) Solid copper (II) oxide reacts with carbon monoxide gas to form solid copper and carbon dioxide gas. D.) Liquid pentene (C5H10) burns in oxygen gas to form carbon dioxide gas and water vapor. E.) Solid iron(III) sulfide is oxidized by oxygen gas to solid iron(III) oxide and sulfur dioxide gas.
Answers
A.) Sodium metal reacts with liquid water to form hydrogen gas and aqueous sodium hydroxide.
2Na(s) + 2H2O(l) → H2(g) + 2NaOH(aq)
B.) Solid phosphorus reacts with chlorine gas to form solid phosphorus pentachloride.
P4(s) + 10Cl2(g) → 4PCl5(s)
C.) Solid copper (II) oxide reacts with carbon monoxide gas to form solid copper and carbon dioxide gas.
CuO(s) + CO(g) → Cu(s) + CO2(g)
D.) Liquid pentene (C5H10) burns in oxygen gas to form carbon dioxide gas and water vapor.
C5H10(l) + 8O2(g) → 5CO2(g) + 5H2O(g)
E.) Solid iron(III) sulfide is oxidized by oxygen gas to solid iron(III) oxide and sulfur dioxide gas.
4FeS(s) + 7O2(g) → 2Fe2O3(s) + 4SO2(g)
In each equation, the reactants are on the left side of the arrow, and the products are on the right. The phases of each substance are included in parentheses after the formula, with (s) representing a solid, (l) representing a liquid, (g) representing a gas, and (aq) representing an aqueous solution. Conditions such as temperature and pressure are not included in the equations.
Learn more about reactants here:
brainly.com/question/14225536
#SPJ11
A 6 kg rock rolls down a hill with a momentum of 12 kg m/s. Work out the velocity of the rock.
Answers
momentum = mass x velocity
When know that:
momentum is 12 km m/s
Mass is 6 kg
Hence, velocity = 12/ 6 = 2m/s
Temperature is the measurement of how hot or cold something is. Agree Disagree
Answers
Answer:
Agree
Explanation:
Answer:
Agree
Explanation:
How many silver atoms are there in 3. 86 g of silver?.
Answers
Answer:
How many atoms are there in 1g of silver approximately? The atomic weight of silver is 108. Thus number of moles of silver in 1 gram is 1÷108=0.009 mole
Explanation:
lead has a density of 11.4 g/cm^3 . what is the density in kilograms per cubic meter in scientific notation?
Answers
Answer:
d = 11400 kg/m³
Explanation:
The density of lead is 11.4 g/cm³
We need to convert the density in kg/m³ in scientific notation.
We know that,
1 kg = 1000 g
1 cm = (1/100) m = 0.01 m
Density,
\(d=11.4\ \dfrac{g}{cm^3}\\\\=11.4\times \dfrac{(\dfrac{1}{1000})\ kg}{(0.01\ m)^3}\\\\=11400\ kg/m^3\)
So, the density of the lead is 11400 kg/m³.
A type of matter formed by chemical joining two or more elements is known as ________.
Answers
a compound is a substance made up of two or more different chemical elements combined in a fixed ratio! :)
Answer: compound
Explanation: i’m right.